State of the Field: PRA 2025 Update
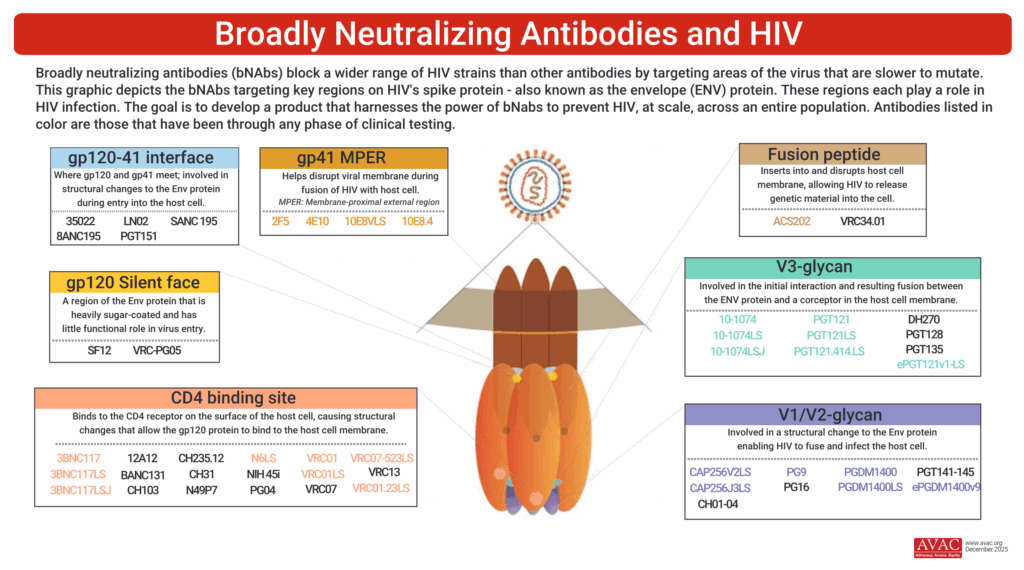
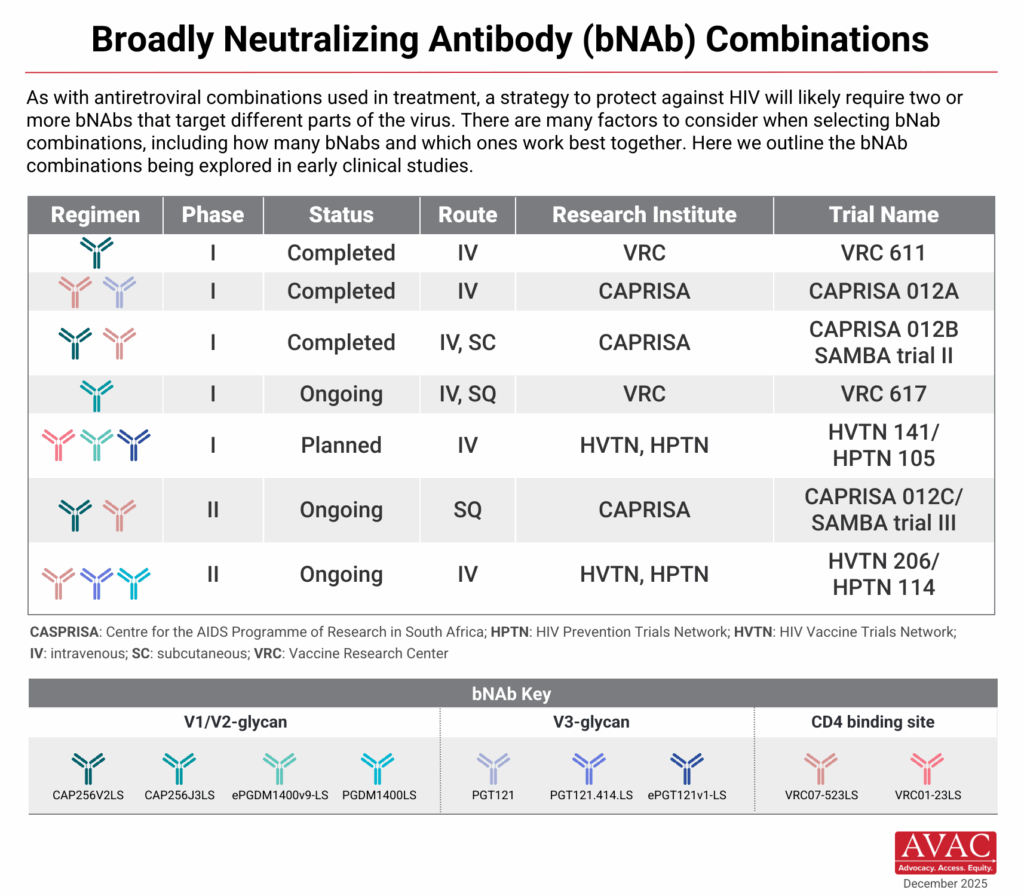
In 2025, researchers continue to advance the concept of “combo AMP,” i.e., passive delivery of a combination of bNAbs targeting multiple sites on the viral envelope, achieving broader and stronger protection than with a single bNAb. The single VRC01 bNAb was a proof-of-concept but showed limited efficacy in the AMP studies, protecting only against highly susceptible viruses, underscoring the need for improved potency, breadth, and duration of protection.
- Next-generation bnAbs continue to be tested in phase I trials primarily run by HVTN and CAPRISA. These bNAbs have been modified to be longer-acting than first-generation bNAbs, and the pipeline now includes a multitude of bNAbs that target various envelope epitopes, as opposed to only the CD4 binding site, targeted by VRC01. These studies are assessing safety, pharmacokinetics, and combination potential to optimize protection and inform future prevention use cases.
- Infant prophylaxis remains a key area of interest in the bNAb field, with a planned Phase 1 study evaluating multiple antibodies in breastfeeding infants to generate critical data for integrating early protection into maternal and child health programs and expanding HIV prevention options for infants.
- During the evolving AMP results and the encouraging laboratory science looking at combinations of bNAbs, significant work has been put into defining a market, manufacturing possibilities, and global access issues for a bNAb product. But translating bNAb research into actual product development and real-world prevention still must address significant questions around delivery (i.e., suggested frequent IV infusions or injections, when ARVs are moving to six and 12-monthly dosing); complex manufacturing of multiple bNAbs into single formulations, and the absence of a clear regulatory or access pathway for prevention or pediatric indications.
- Antibody-based research is critical to inform vaccine design and development, but as discussed in the Preventive Vaccines section, the pathway to an antibody-based product needs to be well-coordinated and interrogated further within the context of the evolving and improving PrEP landscape, and the simultaneous decline in resources.
Click to enlarge
2025 Gaps and Priorities: bnAbs
GAP 1: Unclear role and value proposition within the HIV prevention toolbox
Priority Actions
- Building on work already completed to define a market and use case for passive administration of bNAbs, draw a stronger consensus within the field on where bNAbs provide a unique benefit, such as infant prophylaxis, postpartum prevention, or among populations facing adherence barriers to oral or injectable PrEP.
- Similar to vaccines and underscoring a priority outlined in the original 2024 PRA, develop an updated TPP that reflects the evolving landscape of HIV prevention.
- Ensure that previous work to map manufacturing, delivery, and cost of a potential bNAb product reflects the new reality of HIV prevention, given drastic changes to key players such as PEPFAR.
Click to enlarge
GAP 2: Unclear product development and commercialization pathways for bNAbs, including alignment with feasible use cases and access goals
Priority Actions
- As a bNAb product advances through efficacy testing, develop and implement clear stakeholder engagement plans, with strong emphasis on civil society engagement.
- Clarify the path for product development, including volumes, doses, and administration modes, both that are being tested and that are being aimed for in an ultimate product that could be feasibly distributed and accessed, per an agreed use case and TPP (as articulated above).
- Clearly define the need for and role of a commercial partner in developing a bNAb product, as well as the viability of securing one, and the risks of not.
- Facilitate critical discussions, especially with civil society, around the realities of choosing a bNAb product as a prevention option, especially in the face of improving PrEP options and access, and a more limited ability to offer a multitude of prevention choices.
Navigation
⮐ Return to the 2025 People’s Research Agenda
Previous Edition of the People’s Research Agenda
