State of the Field — PRA 2025 Update
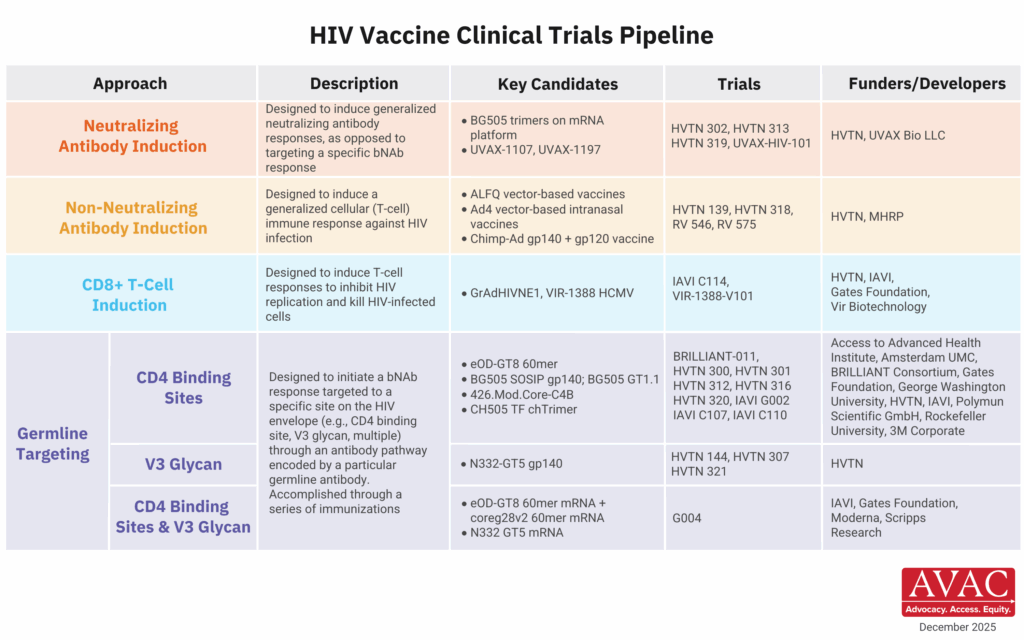
During the current year, HIV vaccine R&D is actively focused on early-stage research, with the portfolio centering primarily on small, iterative discovery medicine trials to develop germline-targeting candidates to induce bNAbs, as well as Phase I trials testing T-cell-inducing candidates. This approach is understandable given recent lessons from efficacy trials which have informed this shift in strategy from traditional product development to the discovery approach to develop a vaccine candidate with promise to induce both bNAbs and T-cell responses.
- In May 2025, key signals read out from IAVI G002/G003 germline-targeting studies, showing initial proof of concept that a series of iterative immunizations could set the immune system on the path to developing bNAbs.
- IAVI initiated a trial in July to test a candidate using a gorilla adenovirus vector, or GRAdHIVNE1, meant to induce CD8 T-cells. Another vector-based program, based on the Cytomegalovirus or CMV vector, continues in its Phase I trial conducted by HVTN in partnership with Vir Biotechnology.
- The HVTN has initiated seven new trials since the PRA launch in 2024, primarily based on the germline-targeting approach. These, and other ongoing programs from key groups such as the US Military HIV Research Program (MHRP), seem to have the field on a renewed path toward identifying a successful HIV vaccine candidate.
- Of major significance was the termination of both the ADVANCE and BRILLIANT vaccine development investments from USAID. Both programs were focused on advancing a range of vaccine candidates while simultaneously investing in African-led vaccine research infrastructure and agendas.
The two NIH-funded Center for HIV/AIDS Vaccine Development (CHAVD) consortia, which drive much of the basic science efforts behind these vaccine candidates, faced initial threats of being entirely defunded by the US government in May. The U.S. administration has also threatened to cut funding for mRNA-based vaccine development, a cornerstone of the HIV vaccine pipeline, following a politicized misinformation campaign. Although the threats to the CHAVDs have since been rescinded, the future of HIV vaccine science faces its most significant risk yet, despite highly encouraging scientific progress.
2025 Gaps and Priorities
GAP 1: Lack of an updated, field-wide Target Product Profile (TPP) for HIV vaccines in the context of evolving PrEP landscape
Priority Actions
- Convene key scientific, policy, and community stakeholders to develop and widely socialize a new consensus HIV vaccine TPP that reflects the current prevention landscape, including the impact of long-acting injectable PrEP, and sets clear expectations for durability, affordability, simple dosing, and equitable delivery.
- Use the TPP as the anchor for all HIV vaccine advocacy and funding dialogues, ensuring coherence across the field. Eliminate fragmented advocacy efforts that are not grounded in the TPP.
- Keep HIV vaccines firmly on the global research agenda by emphasizing their unique contribution to a durable, sustainable end to HIV.
- Ensure that HIV vaccines are positioned as additive tools within a choice-based prevention toolkit.
GAP 2: Lack of a coordinated vaccine research roadmap that clearly articulates interim milestones of success
Priority Actions
- Develop a comprehensive global roadmap for HIV vaccine R&D, mapping current scientific strategies (germline targeting, T-cell-based, mRNA approaches), anticipated interim milestones, and pathways to a candidate that meets a consensus TPP.
- Continue sustained investment in HVTN, IAVI, MHRP, BRILLIANT, the CHAVD consortia, and other vaccine research sponsors and institutions to support upstream science, translational research, and coordinated trial implementation.
GAP 3: Weakening political and public support
Priority Actions
- Refresh targeted messaging that communicates the broad and collateral benefits of HIV vaccine research, including mRNA platform advances, contributions to pandemic preparedness, and the fundamental public health value of vaccines.
- Based on the work described above on a coordinated vaccine research roadmap, articulate in lay language the promise and importance of developing an HIV vaccine, while also being transparent about the highly challenging, long-term, and iterative nature of the science.
- Invest in community-led education, communication, and advocacy to counter misinformation and disinformation, build trust, and address vaccine hesitancy in diverse communities.
Resources
- HIV Prevention at a Crossroads: Why we still need an HIV vaccine
- From The Lab To The Jab series
- HIV Vaccine Awareness Day webinar recording
- PxPulse podcast with Nina Russell
Navigation
⮐ Return to the 2025 People’s Research Agenda
Previous Edition of the People’s Research Agenda
