Contacts
Mitchell Warren, [email protected], +1-914-661-1536
Kay Marshall, [email protected], +1-347-249-6375
AVAC says donors, researchers, regulators and communities must act swiftly to determine future of this approach
Boston, Massachusetts — Results of two large-scale clinical trials in Africa show promise for a potential new HIV prevention option for women but more work is needed before the vaginal ring containing the antiretroviral (ARV) drug dapivirine can be added to the very short list of HIV prevention options controlled or initiated by women.
“It’s clear that the dapivirine vaginal ring can be a viable option for women to protect themselves from HIV,” said Mitchell Warren, AVAC executive director. “These well conducted studies provide clear evidence that some women can reduce their risk of acquiring HIV by using the ring consistently, and they also raise critical questions about the level of use, benefit and future role in prevention that need to be answered. Researchers, donors, regulators and advocates now need to determine the best way forward to determine if and how the dapivirine ring could be used by women at risk of HIV infection.”
“Many women in sub-Saharan Africa – and notably younger women – remain at substantial risk of contracting HIV, and they need and deserve a range of options that they can control and comfortably use to protect themselves every time they have sex. Today, daily oral PrEP is the only truly discrete prevention option they can control. The dapivirine vaginal ring might become an additional option, as additional questions are answered and regulatory agencies consider these results. In the meantime, the incredibly high HIV infection rates among women in these trials tell us that we need to make oral PrEP more widely accessible and available with urgency.”
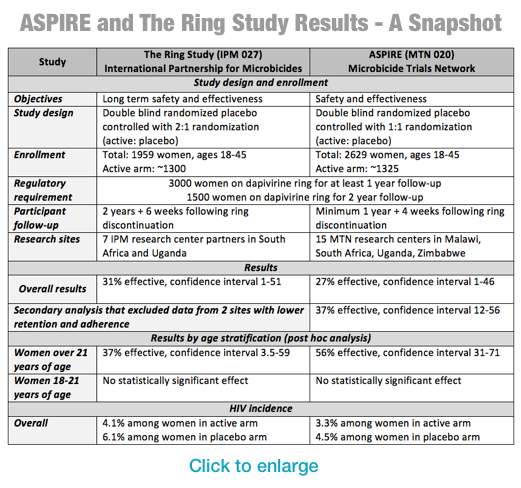
The results of the two studies, ASPIRE and The Ring Study, were previewed today by researchers at the Conference on Retroviruses and Opportunistic Infections (CROI) ahead of a scientific presentation later this week. Results from the ASPIRE study were also published today in the New England Journal of Medicine. The trials evaluated the safety and efficacy of a vaginal ring – which women placed themselves and replaced each month – containing the ARV dapivirine.
Both trials had similar results. Among all women in the ASPIRE study, the ring reduced the risk of transmission by 27 percent; in the Ring Study, the overall reduction was 31 percent. Both studies found the ring was safe to use.
In both trials, efficacy was substantially higher among the subset of women who were over 21 who appeared to keep the ring in consistently throughout the month. As with previous trials with other prevention methods, adherence to the prescribed use appeared higher among older women, which might explain higher levels of efficacy in these age groups. (For more information on the results see the table at the end of release).
“We congratulate the trial sponsors, scientific collaborators and partners for these well run and scientifically rigorous trials. We especially want to thank the more than 4,500 women whose altruism and commitment as trial participants made this effort possible,” Warren added.
The lack of protection seen among women in the trial between 18 and 21, likely because of lower adherence, is not unique to these two studies. Previous ARV-based prevention efficacy trials with both tenofovir gel and tenofovir-based oral PrEP also saw lower protection among younger male and female participants, likely due to lower adherence. With daily oral PrEP, however, adherence increased for younger participants in subsequent post-trial studies, in which participants knew that PrEP had already been proven effective and that they were receiving the active product.
“It is reasonable to believe that both adherence and efficacy can be increased for dapivirine ring use, just as we’ve seen it increase over time in PrEP studies,” Warren said. “A critical first step is to launch open label extension studies, with no placebo, among former ASPIRE and Ring Study participants. These studies results are needed to help guide decisions about regulatory approval and, perhaps, eventual rollout of a monthly dapivirine ring. In addition, we need to look at streamlining the process of developing a combination ring that would could protect against both HIV and pregnancy.”
As the product developer, IPM, along with the other trial sponsors and researchers, look at the path forward for the dapivirine ring, they can look to learn lessons from oral PrEP programs, female condoms and from the contraceptive field to understand how to better support adherence, improve product use and address special access needs of young women.
“HIV continues to be a public health crisis, especially for young African women who have few options for protecting themselves from HIV infection,” Warren said. “With each new prevention research result over the past six years show partial protection – for oral PrEP, microbicide gel and the RV 144 vaccine – AVAC said there was a global imperative to act on those results,” Warren said. “Today, there is a global imperative to act on these new vaginal ring results with a clear path to regulatory review and a fully funded, carefully prioritized research agenda to help answer the remaining questions about the dapivirine ring and how it might be added to prevention options for women.”
“While we need understand the path forward for rings and deliver existing HIV prevention and treatment options, we also need to press ahead with research and development of additional options. As long as women continue to be infected by HIV at high rates, we have a moral imperative to sustain the search for prevention options that women will want and use, including long-acting ARV-based prevention options, vaccines, antibody-mediated prevention and multipurpose prevention options that may combine HIV prevention with contraceptives,” Warren said.
###
About AVAC: Founded in 1995, AVAC is a non-profit organization that uses education, policy analysis, advocacy and a network of global collaborations to accelerate the ethical development and global delivery of AIDS vaccines, male circumcision, microbicides, PrEP and other emerging HIV prevention options as part of a comprehensive response to the pandemic.