Resources
AVAC’s Resource Database contains educational and advocacy materials covering a wide range of issues on biomedical prevention of HIV, STIs, COVID-19 and emerging health threats—from research to rollout.
To search for clinical trials and detailed information on products in development, visit our Prevention Research & Development Database

Results
showing 1-10 of 984
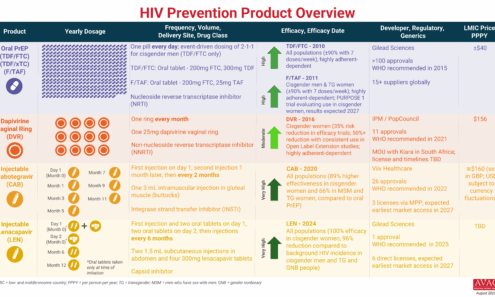
HIV Prevention Product Overview
The graphic provides an overview of PrEP products currently available and in late-stage clinical trials.
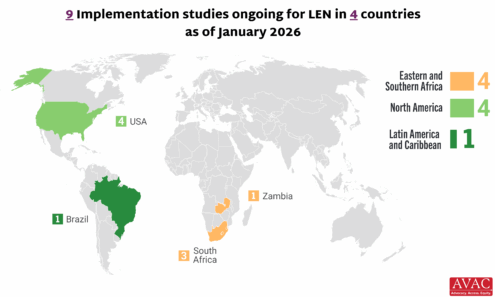
Lenacapavir Implementation Studies
Ongoing and planned implementation studies for the lenacapavir as of January 2026.
Prevention Option:
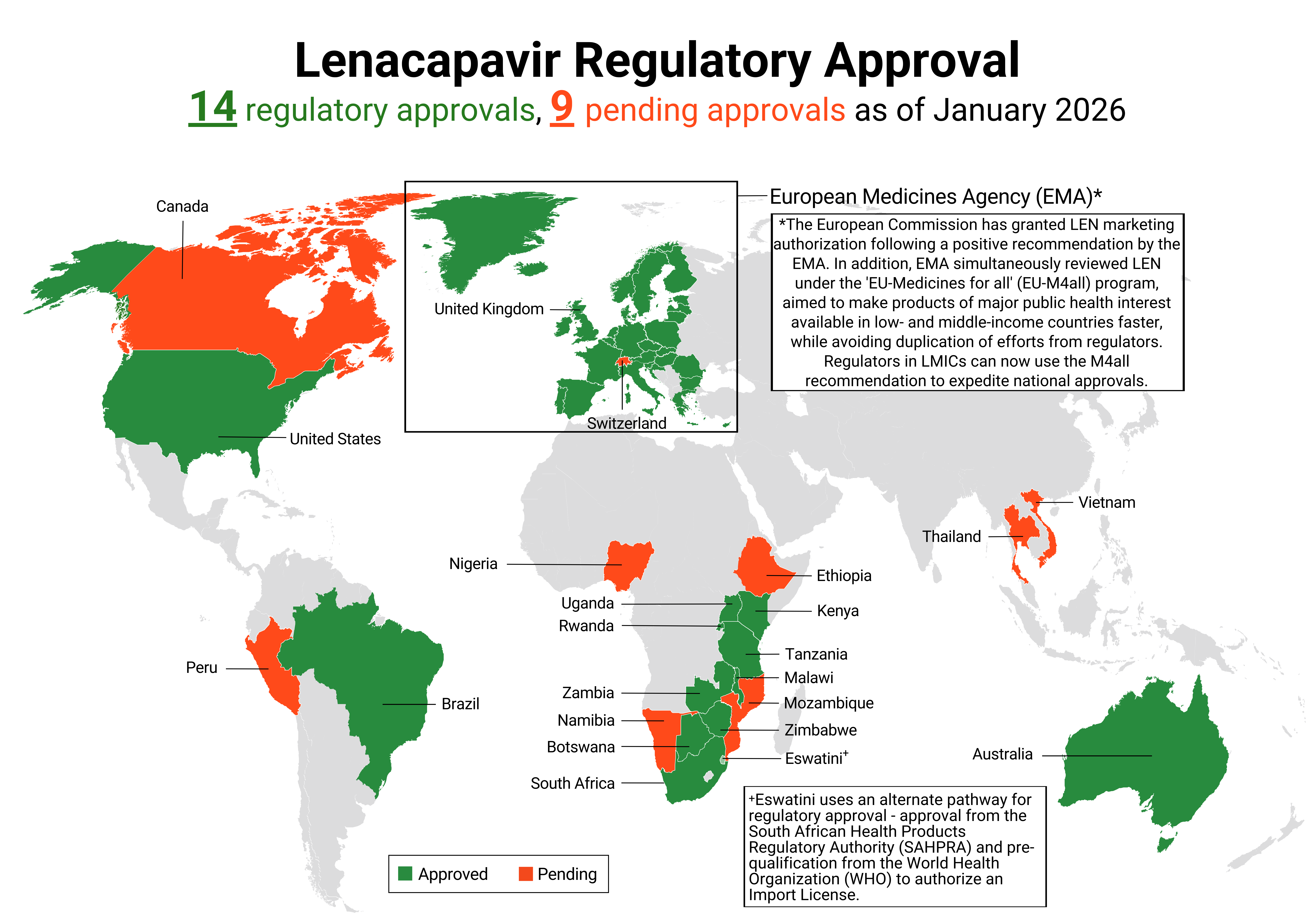
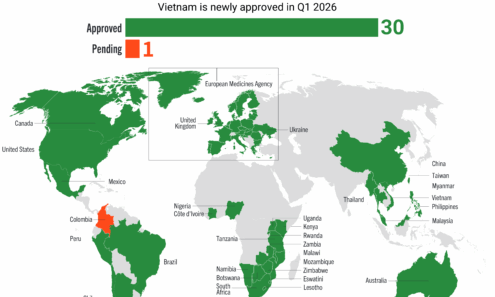
Lenacapavir Regulatory Approval
Regulatory approvals, pending decisions, and appeals as of January 2026.
Prevention Option:
Cabotegravir Regulatory Approval
Regulatory approvals and those pending for cabotegravir as of January 2026.
Prevention Option:
HIV Vaccine Clinical Trials Pipeline
This graphic summarizes the state of HIV vaccine research, detailing the different immunological approaches in clinical trials, the specific candidates being studied, and the collaborative networks of funders and developers working toward an effective vaccine.
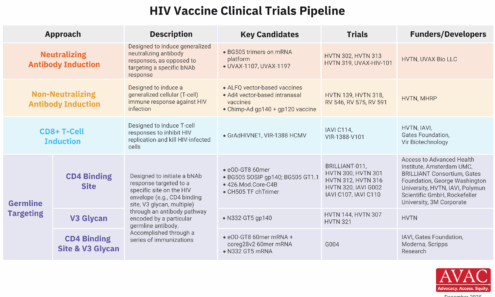
EXPrESSIVE Phase 3 Trials Countries of MK-8527
Seventeen countries are hosting sites for the Phase 3 efficacy trials of a monthly PrEP pill, MK-8527, being developed by Merck (also known as MSD outside of the US and Canada). Merck announced the launch of the Phase 3 trials at IAS 2025 in Kigali. MK-8527 was found to be safe and well-tolerated in Phase 2 clinical trials.
Prevention Option:
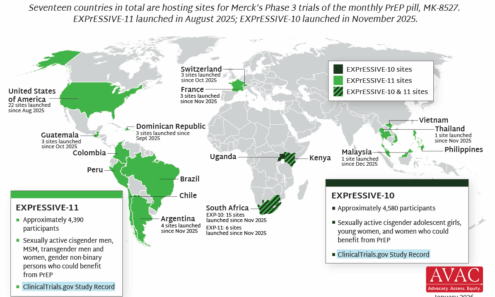
HIV Prevention R&D at Risk
AVAC’s analysis of the impact of US Government funding cuts, terminated projects, and other policy changes on the HIV prevention research and development (R&D) pipeline, and on HIV research broadly.

STIWatch Quarterly Newsletter
As many global health fields reassess their reliance on US government funding for research and development, the STI field—already underfunded and reliant on alternate donors—now faces even greater uncertainty. In this newsletter, we share a new STI resource for advocates and highlight the top issues we’re monitoring as events continue to unfold.
People’s Research Agenda
The People’s Research Agenda sets out a people-centered framework for equitable and accelerated R&D and product introduction. It tracks the science, shows where investments align—or fail to align—with community-defined priorities, and spotlights critical gaps in the pipeline of prevention options needed to meet the diverse realities of all populations.
Prevention Option:
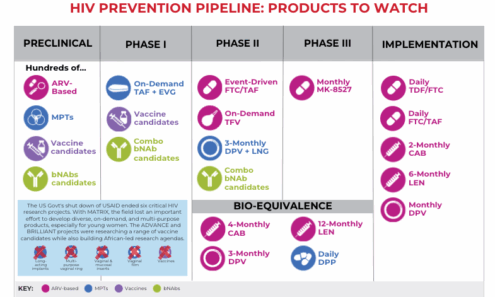
Multipurpose Prevention Technologies (MPTs) Studies Halted by USAID Funding Termination
This graphic shows a list of which studies have been halted by eliminated USAID funding.
showing 1-10 of 984