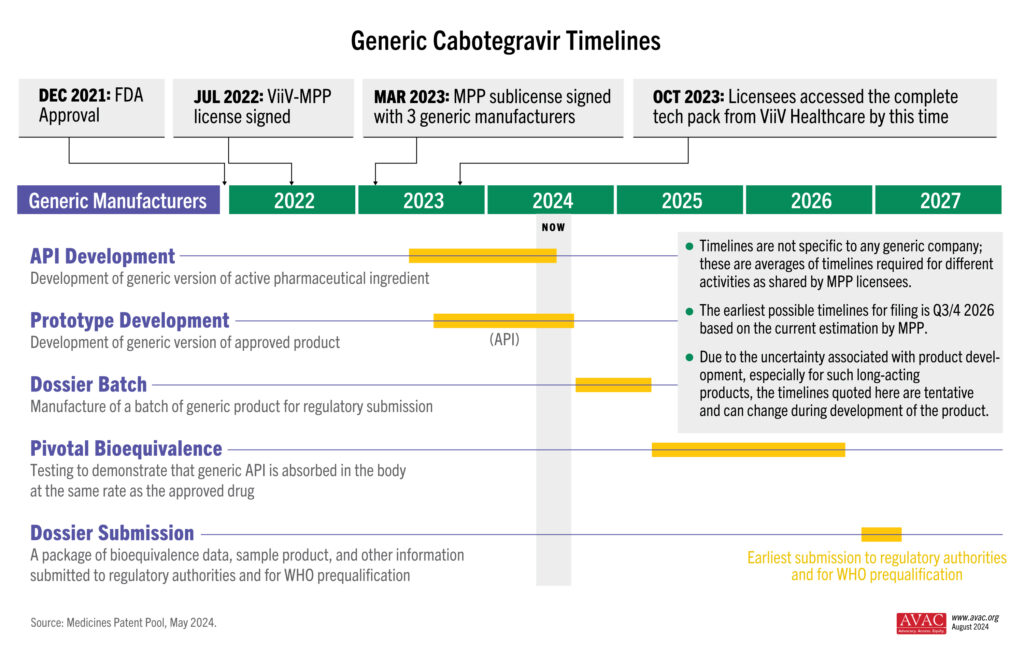
As CAB for PrEP is a long-acting, extended-release injection, bioequivalence (BE) testing takes time to determine whether the generic drug functions in the body similarly to the original drug. As shown in this timeline graphic, the BE study (as determined by WHO guidance) is the longest part of the development process for generic CAB. But other steps, such as selecting and licensing generic manufacturers and technology transfer could be done faster.
Was this content helpful?
Tell us how we can improve the content.
Was this content helpful?
Thank you for your feedback!