PxWire Volume 14, Issue No. 4
Takeaways
- Since 2019, rates of HIV acquisition in Latin America have been trending upward. At the same time, Latin America has taken strides to combat this trend, from increasing PrEP initiation rates to preparing for longer acting PrEP products.
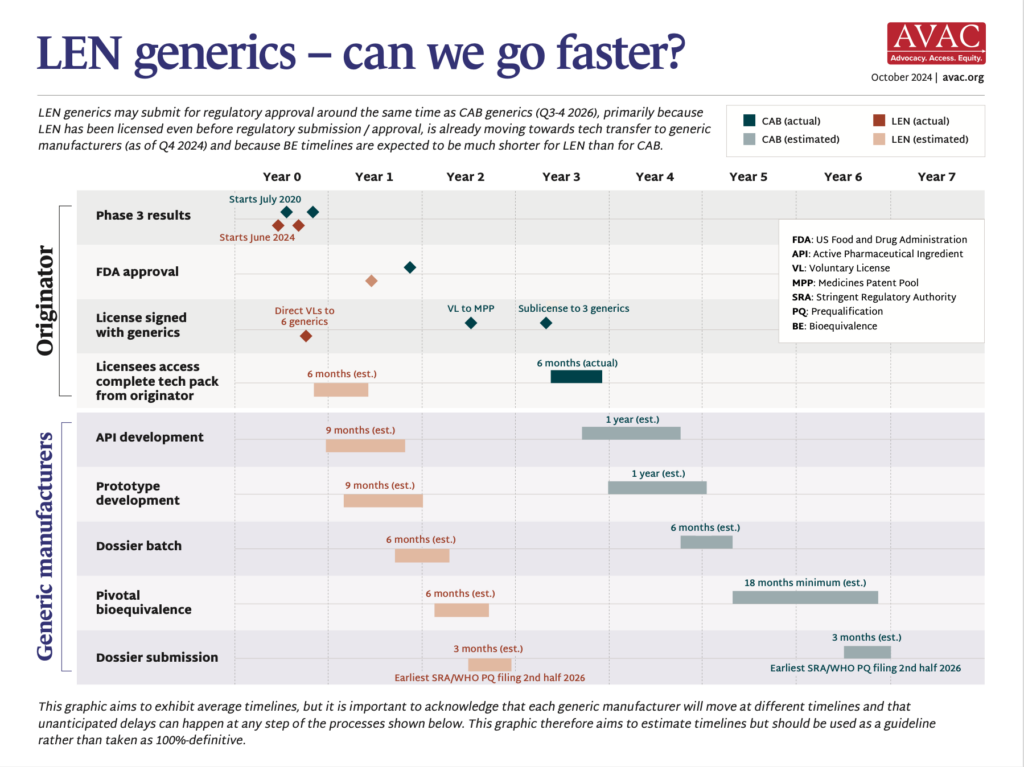
- On October 2, Gilead Sciences announced six licenses to generic manufacturers in Egypt, India, and Pakistan to produce and market lenacapavir for PrEP in
120 countries. - Generic regulatory approval submission for LEN for PrEP may begin as early as the second half off 2026, two years after Gilead announced positive trial results.
- Launched at HIVR4P 2024, the People’s Research Agenda is a collaboratively developed framework for aligning the needs and priorities of affected communities with the agenda for HIV prevention research.
PxWire is AVAC’s quarterly update covering the latest in the field of biomedical HIV prevention research and development, implementation and advocacy. Each issue includes updates, emerging issues and upcoming events. A PDF version of this report is also available.
Progress in PrEP Uptake
The HIVR4P 2024 conference, hosted this year in Lima, Peru, put a spotlight on PrEP delivery in Latin America. Since 2019, rates of HIV acquisition in the region have been trending upward, from 110,000 annually in 2019 to approximately 120,000 in 2023. At the same time, Latin America has taken strides to combat this trend, from increasing PrEP initiation rates to preparing for longer acting PrEP products, such as injectable cabotegravir (CAB) and lenacapavir (LEN).
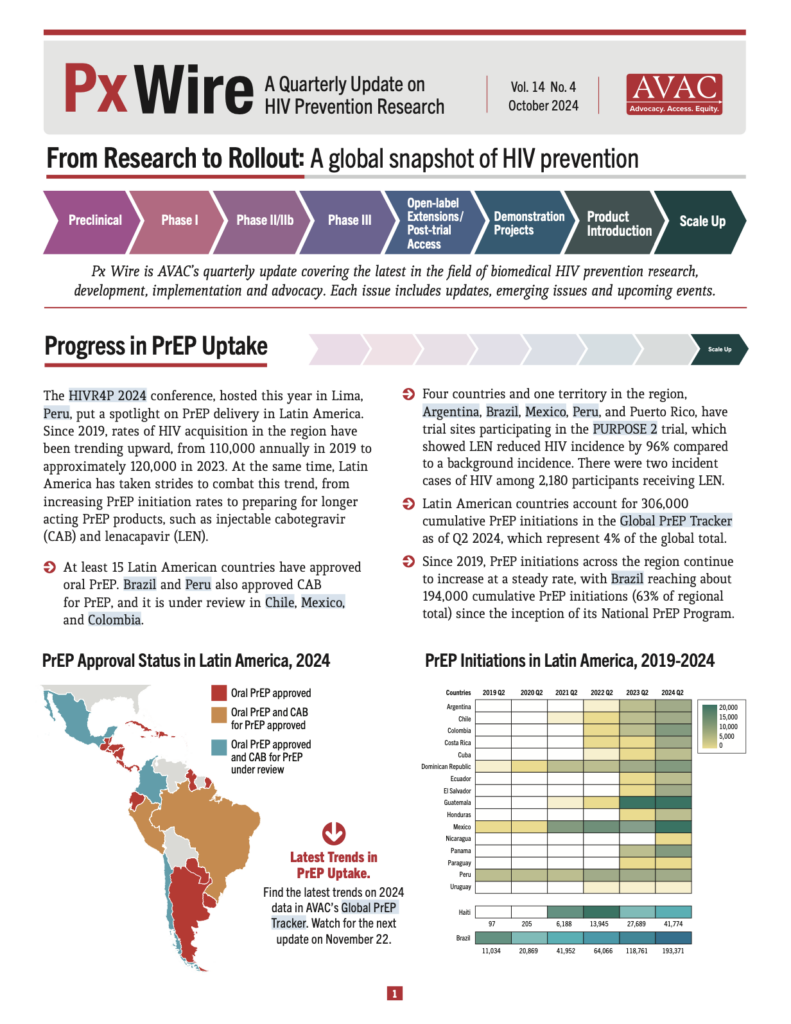
- At least 15 Latin American countries have approved oral PrEP. Brazil and Peru also approved CAB for PrEP, and it is under review in Chile, Mexico, and Colombia.
- Four countries and one territory in the region, Argentina, Brazil, Mexico, Peru, and Puerto Rico, have trial sites participating in the PURPOSE 2 trial, which showed LEN reduced HIV incidence by 96% compared to a background incidence, with 2 incident cases among 2,180 participants receiving LEN.
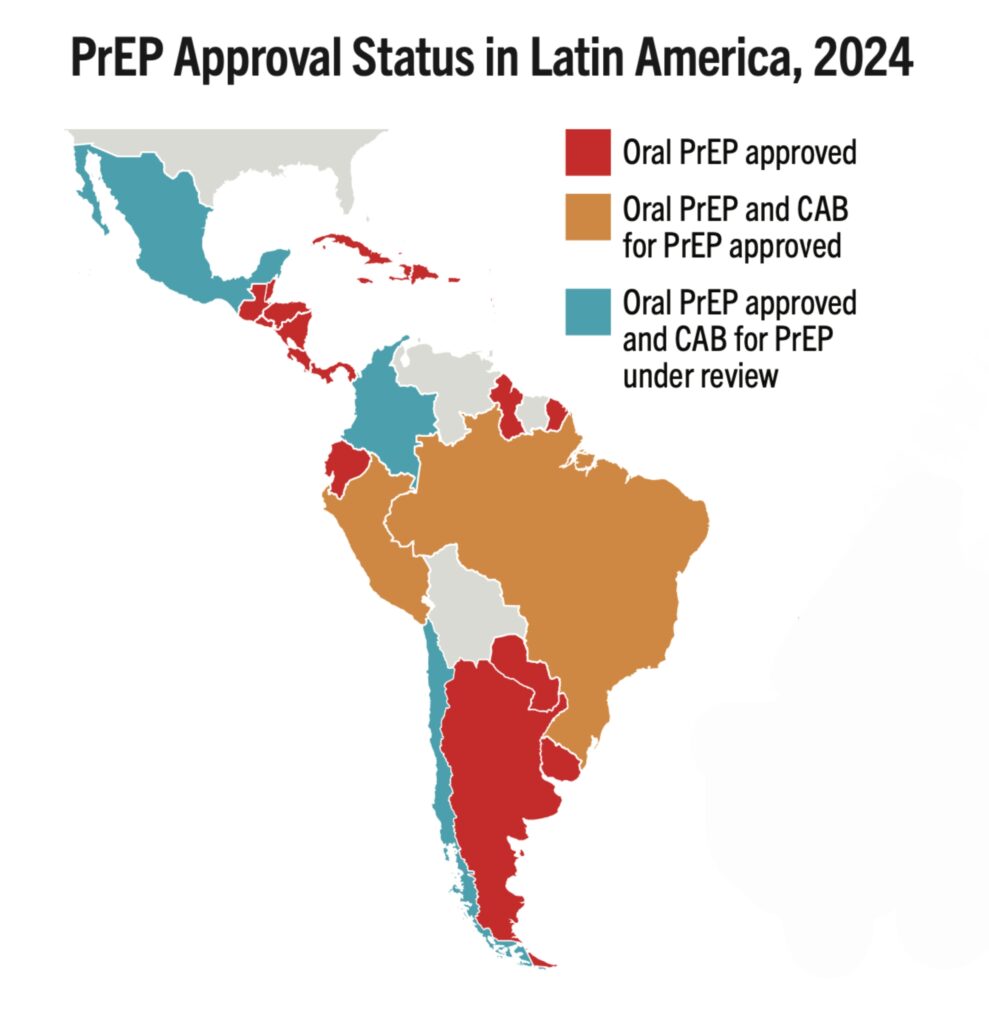
- Latin American countries account for 306,000 cumulative PrEP initiations in the Global PrEP Tracker as of Q2 2024, which represent 4% of the global total.
- Since 2019, PrEP initiations across the region continue to increase at a steady rate, with Brazil reaching about 194,000 cumulative PrEP initiations (63% of regional total) since the inception of its National PrEP Program.
Latest Trends in PrEP Uptake?
Find the latest trends on 2024 data in AVAC’s Global PrEP Tracker. Watch for the next update on November 22.
PrEParing for New Products
- On October 2, Gilead Sciences announced six licenses to generic manufacturers in Egypt, India, and Pakistan to produce and market lenacapavir (LEN) for PrEP in 120 countries.
- What’s new about this announcement? This marks an increase in speed, as compared to generic licenses for injectable CAB for PrEP. Gilead awarded these licenses even before submitting LEN for PrEP for regulatory approval in any country. Gilead’s license applies to 120 countries, which is more than the 90 countries covered by the generic license for CAB for PrEP. However, CAB for PrEP’s license also allows for generic supply in countries without patent barriers—by the time generic CAB for PrEP is available, this will be an additional 51 countries. Many countries with high HIV incidence have been left out of Gilead’s licensing deal; notably Argentina, Brazil, Mexico, and Peru, countries in which the PURPOSE 2 Phase III trials took place. In addition, Gilead’s licenses are more restrictive than ViiV’s, in terms of where generics can be marketed.
- The timeline for generic LEN for PrEP to come to market is expected to be significantly shorter than for CAB for PrEP. Bioequivalence (BE) testing for LEN, which demonstrates a generic product works in the body in the same way as the originator product, is likely to be six months, versus the 18 months for CAB for PrEP, because of differences in the drug formulation. The rapid granting of voluntary licensing by Gilead also contributes to this shorter timeline.
- Generic regulatory approval submission for LEN for PrEP may begin as early as the second half off 2026, two years after Gilead announced positive trial results. Generic CAB for PrEP is on track to submit for regulatory approval around the same time, approximately six years after the announcement of trial results.
Product Updates
- CAB for PrEP has been approved in Cote d’Ivoire, Kenya, Myanmar, Namibia, and Ukraine. See AVAC’s Planning Matrix for more!
The Latest R&D in the Prevention Pipeline
Launched at HIVR4P in Lima, Peru in October 2024, the People’s Research Agenda (PRA) is a collaboratively developed framework for aligning the needs and priorities of affected communities with the agenda for HIV prevention research. 130 advocates from more than 20 countries contributed to the framework, which offers guidance on both research conduct and what products to develop.
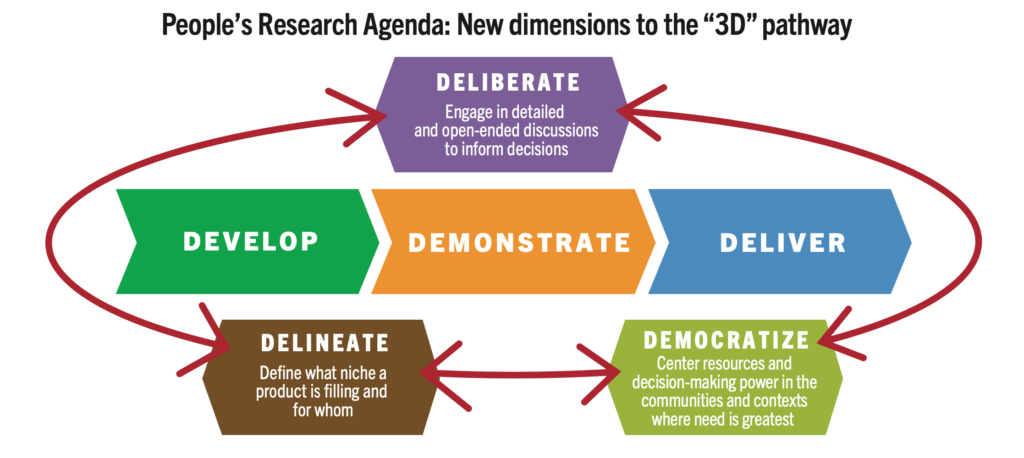
PRA priorities include:
- Support a choice-based prevention agenda with clear and realistic target product profiles that reflect the current and future landscape of HIV prevention. Begin with the end in mind by identifying and anticipating trial design issues (feasibility, cost, size, regulatory pathways) and implementation science questions so that the timeline from evidence to impact and to widespread access is as short as possible. Initiate access planning during efficacy trials to ensure expeditious rollout.
- Center and invest in social and behavioral science that explores end users’ needs and preferences, informs target product profiles, and identifies and addresses social and structural barriers in HIV prevention.
- Maintain advocacy across product categories to fill important gaps in a comprehensive HIV prevention toolbox. We must make newly efficacious products accessible, and vaccines, bnAbs, implants, and other products in early-phase development will also play an important role for a sustainable end to the epidemic.
Key considerations:
- Invest in continuous learning so that advocates and communities where research is planned are equipped to weigh in on complex and evolving trial designs and approaches.
- Novel trial designs using external controls are important in developing new prevention products. However, they must meet the rigorous standards of randomized controlled trials (RCTs) to ensure they provide reliable answers to key research questions without compromising quality.
- Trial designs must address the needs and concerns of highly impacted communities.
- Products moved into trials should be ones that fill community-informed gaps, such as improving upon existing prevention methods with innovations in cost, mode of delivery, duration or level of protection and other parameters.
- Participants should be supported to clearly understand how novel design elements, such as run-in or opt-out phases, may impact their involvement.
- Ensure that regulators are prepared to review and react to the data generated by future trials, especially if it is a novel design.
The PRA is a living document—emerging priorities, challenges and opportunities will be reflected in ongoing updates. Please share your thoughts, reflections, and comments by emailing Grace Kumwenda at [email protected]. For more information on the People’s Research Agenda visit avac.org/peoples-research-agenda.
Prevention Playlist
AVAC develops a wide range of resources to inform decision making and action. Check out the latest:
Join
- An Update on the STI R&D Pipeline and Investments, November 14
- True Choice in HIV Prevention Involves More than Product Options: Novel strategies in service delivery, November 19
- Lens On LEN, a discussion with Gilead, December 3
Use
- People’s Research Agenda, Report
- Moving a Product to the Real World
- From Clinical Trial Efficacy to Public Health Impact: A Plan for Accelerating Access to Injectable Lenacapavir for PrEP
- An Overview of Lenacapavir for PrEP Trials
- The Lens on LEN: The Basics on Injectable Lenacapavir as PrEP
- The Future of ARV-Based Prevention and More
- Spotlight on MPTs Addressing STIs
- Advocates’ Guide to Multipurpose Prevention Technologies (MPTs)
- Sexual and Reproductive Health Integration Advocacy Roadmap
- Explore STIWatch.org and get the latest resources to navigate the IUSTI & STI Prevention meetings!
Watch and Listen
- PxPulse Podcast Lenacapavir: The case for investing in delivering HIV prevention
- Breaking New Ground: Expanding Access to Lenacapavir—Lessons from Dolutegravir and the Future of HIV Prevention
- Let’s Talk LEN: What global advances in HIV prevention mean for Black communities in the US
- Launch of CIFF/Global Fund Initiative for the PrEP Ring
- Integrating HIV and PrEP Services in US Correctional Facilities
- PIBA Presents Reimagining the HIV Prevention Blueprint for Black Communities in Arkansas, Mississippi, and Tennessee
- From Promise to Progress: Overcoming Barriers to Long-Acting PrEP Uptake among Black Gay Men in the US
- PxPulse: The Advocacy Chronicles with Danielle Campbell from PrEP in Black America
- Vaccinology of HSV
- The Road Ahead—SRH Integration Advocacy
- Do Vaginas Demand Perfection? Implications for Event-Driven PrEP
- Restrategizing Civil Society Engagement for Pandemic and Global Governance
- On the Frontlines of AMR: A Systems Approach
- Sustainability of the HIV/AIDS Response—Getting to 2030 & Beyond
- Innovations in GPP
- PrEP Your Booty – The Launch of HPTN 106 “Rev Up”
- Opportunities to Expand Equitable Access to HIV Prevention Services through Community Pharmacies
- PxPulse: An Advocacy Chronicle on U=U in South Africa with Mandisa Dukashe
Read
- AVAC Calls on Gilead and Global Stakeholders to Accelerate Access to Generic Lenacapavir Following License Agreements
- Restrategizing Civil Society Engagement for Pandemic and Global Governance
- HIV self-testing and PrEP, fighting health misinformation, innovations in GPP, and more!
- HIV leaders say it’s time to end the long wait for long-acting products
- What’s Next for the Dual Prevention Pill
- Statement of Solidarity with the Nigerian Key Population Community on the Murder of their Leader, Christopher Ikpu Terfa: A Call for unity, solidarity, and government protection
- Pandemic Accord Priority for 2025
- Harnessing private sector strategies for family planning to deliver the Dual Prevention Pill, the first multipurpose prevention technology with pre-exposure prophylaxis, in an expanding HIV prevention landscape
Was this content helpful?
Tell us how we can improve the content.
Was this content helpful?
Thank you for your feedback!