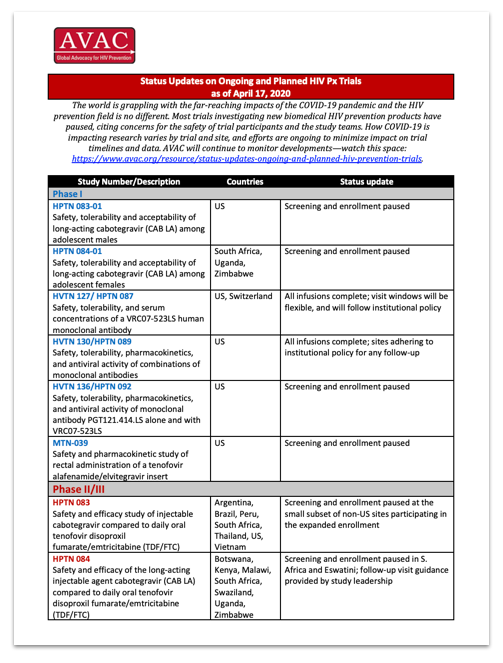
The world is grappling with the far-reaching impacts of the COVID-19 pandemic and the HIV prevention field is no different. Most trials investigating new biomedical HIV prevention products have paused, citing concerns for the safety of trial participants and the study teams.
How COVID-19 is impacting research varies by trial and site, and efforts are ongoing to minimize impact on trial timelines and data. AVAC will continue to monitor developments—watch this space.
Was this content helpful?
Tell us how we can improve the content.
Was this content helpful?
Thank you for your feedback!