In June 2025, the US Food & Drug Administration’s approval of injectable lenacapavir (LEN) provided a much-needed boost for HIV prevention, particularly given the strength of the science and the simultaneous global disruption of HIV research and programs by the US government.
Drawing on three decades of experience and expertise from earlier PrEP rollouts, AVAC served as a leading voice for access and equity, demanding that “scientific progress only matters if innovation actually reaches people. LEN for PrEP is poised to re-shape the HIV response, but only if approval is accompanied by bold, strategic, effective and equitable rollout that reaches the populations that need access.”
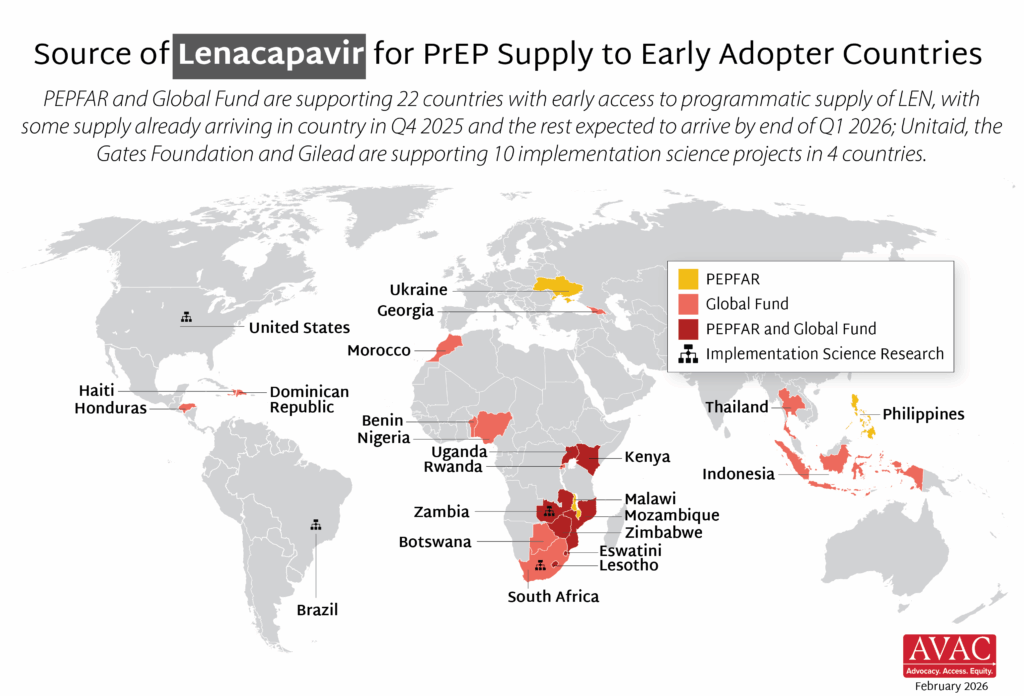
Map showing sources of LEN for PrEP supply across early adopter countries.
AVAC’s efforts helped lay the groundwork for LEN to move faster and more equitably through the pipeline and into roll-out. In 2025, AVAC strengthened the partnerships, coordination and evidence base needed for rapid and inclusive LEN scale-up. Six key LEN milestones were achieved in record time; what took more than five years for oral PrEP and two years for injectable CAB following the publication of efficacy results, took just a year for LEN—including multiple regulatory approvals, WHO recommendation and pre-qualification, and initial product launch.
Over the years, AVAC has served as a translator, catalyst and advocate for the field, accelerating PrEP product development and introduction. AVAC’s online PrEPWatch clearinghouse tracks global progress, challenges and accountability across PrEP research, access and rollout; AVAC convenes stakeholders to advance global conversations on product development and delivery, and to ensure community
advocates have a central voice in decision-making spaces. AVAC’s LEN resources were among the most sought-after and referenced documents in the field—among advocates, Ministries of Health, researchers and implementers—providing evidence and analysis for fieldwide decision-making during LEN rollout planning.
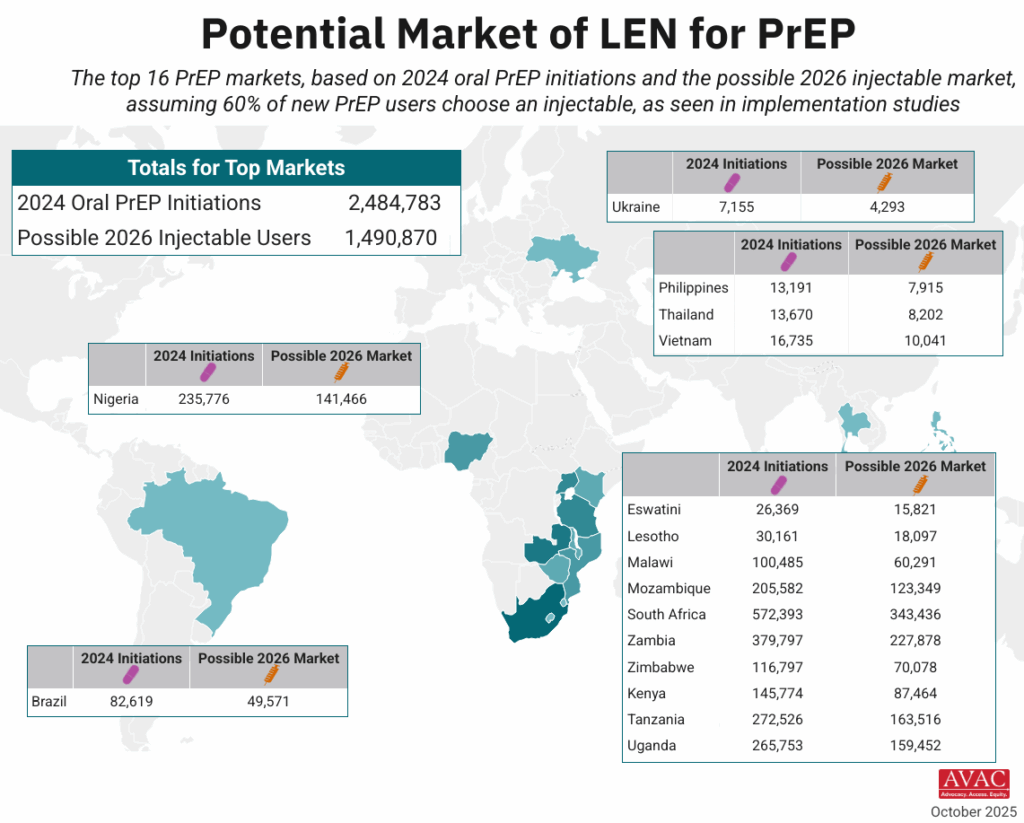
Top 16 countries with the largest project markets for long-acting injectable PrEP.
AVAC’s leadership resulted in the creation of sustainable models for collaboration, including the Coalition to Accelerate Access to Long-Acting PrEP, among normative agencies, funders, civil society, ministries of health and implementers, fostering early national action plans, stronger donor alignment and inclusive strategies for faster LEN rollout. Working closely with country-level stakeholders, AVAC established a Community of Practice for ministries of health as a space for regional learning, donor dialogue and problem-solving. AVAC supported civil society partners in early adopter countries, centering civil society’s leadership, providing a structured foundation for national stakeholder meetings and ensuring accountability and swifter action toward LEN introduction at the country level.
Comparison of LEN milestones after efficacy results, with oral PrEP, DVR and CAB. Static version available here.
AVAC served as the global source for evidence-based materials to support LEN introduction, including infographics, analysis and guides covering regulatory approvals, procurement and pricing updates, and providing key advocacy messages to accelerate scale-up. By producing and sharing timely, accessible information, this work ensured field-wide coordination and enabled more intentional, informed civil society engagement in planning for LEN rollout.