Research on broadly neutralizing antibodies (bNAbs) is taking HIV prevention science in new directions. Passive immunization is one strategy under investigation. See our cure and vaccine pages to learn about bNAb research in these other areas of HIV intervention.
What is a Broadly Neutralizing Antibody?

Antibodies are part of the body’s natural defense system to fight infection. Since HIV is constantly changing and disguising itself, antibodies that attack parts of the virus that are slower to mutate—known as “conserved regions—stand the best chance of mounting an effective defense against HIV. Researchers have found many bNAbs, which block a wider range of HIV strains targeting these conserved regions. The challenge is identifying a strategy that will harness the power of bNAbs in a product that will work at scale, across an entire population, to prevent HIV.
How bNAbs Develop
About 20 percent of people living with HIV naturally develop bNAbs, after many years. By the time bNAbs have developed in these individuals, the constantly mutating HIV has outpaced these defenders, changing too fast and too significantly for bNAbs to be effective in that individual. But that same bNAb, or a combination of them, may work in someone else. Scientists have been able to isolate an increasing number of these bNAbs, copy them, and manufacture them to test how well they prevent HIV acquisition. A bNAb known as VRC01 is an example of this strategy. The Antibody Mediated Prevention Trials (AMP) used VRC01 in the first large-scale trials to show proof-of-concept that bNAbs could potentially be an effective new tool to protect against HIV. See our AMP Trials page for background and resources.
What is Passive Immunization and Why is it Relevant to Research on bNAbs?
Immunization refers to an action that enables the body to fend off infection, often by vaccination. For example, vaccines trigger the body’s immune system to recognize a specific pathogen and mount a defense. The strategy used by the AMP trials did not use the body’s immune system, as vaccines do. Instead, this research relied on “passive immunization”, which involved using an infusion of VRC01 to block HIV from infecting human cells.
This infusion of bNAbs did not trigger the body’s immune system to learn to identify a new pathogen, and infused bNAbs will not last for life. With passive immunization, bNAbs block HIV for as long as they last in the body, a period that is still unknown. Periodic infusions were tested in the AMP trials and may be necessary for future products to sustain protection. How feasible this strategy will be for people in the real world is not clear. Some research is exploring the use of a subcutaneous injection of bNAbs, just under the skin, as passive immunization. How long the protection lasts, in all these strategies, is a crucial scientific question.
Access to bNAbs and Passive Immunization
Ensuring communities where health systems are constrained can readily access a prevention strategy that relies on passive immunization is a key point of advocacy. How to scale up such a product and keep it affordable depends on several factors. These include whether effective bNAbs can be developed as a subcutaneous injection versus the more time-intensive use of infusions. Other factors will determine the feasibility and accessibility of bNAbs globally including local and generic manufacturing, cost, and affordability. bNAbs as passive immunization hold particular promise for the prevention of the transmission of HIV to babies during childbirth and breastfeeding.
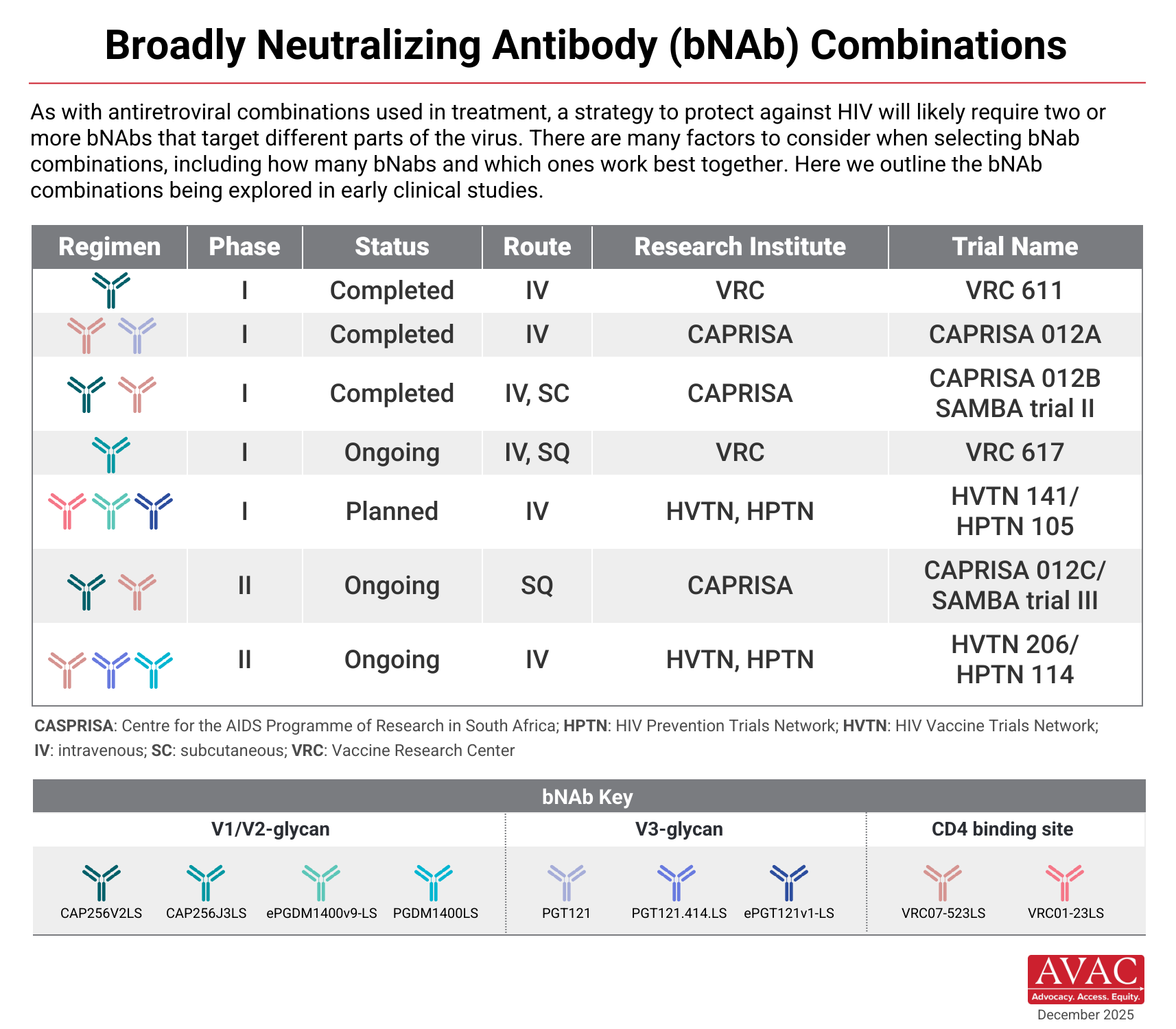
Which bNAbs are Currently in the Pipeline?
Dozens of bNAbs are under investigation for HIV prevention. In February 2021, results of the AMP trials were announced. They showed that VRC01 did not reduce the overall risk of acquiring HIV. However, VRC01 protected individuals who were exposed to strains of HIV that were particularly vulnerable or “sensitive” to the antibody. This is the first evidence in humans that intravenous infusions of a bNAb can reduce a person’s risk of acquiring HIV.
Lessons from the AMP trials indicate that more than one bNAb may be needed to mount an effective defense against HIV. Treatment research, which led to combination antiretroviral therapy (ARVs), offered a similar lesson. Combinations may be key. Many early clinical studies are now testing combination bNAbs using passive immunization. Candidates are being tested as both infusions and subcutaneous injections.
Advocacy and Passive Immunization
Now the task is to consider how a passive immunization strategy could be feasible in the real world and what role this tool could have in the prevention toolkit.
Visit our vaccine and cure pages for more on bNAb strategies in other interventions. HIV vaccines using a bNAb strategy are aimed at speeding up the immune system process of developing bNAbs naturally. Find details on how bNAbs work in this article on AMP Trials by AVAC founder, Bill Snow.
People’s Research Agenda
Our People’s Research Agenda offers a state of the field update on broadly neutralizing antibodies. researchers continue to advance the concept of “combo AMP,” i.e., passive delivery of a combination of bNAbs targeting multiple sites on the viral envelope, achieving broader and stronger protection than with a single bNAb.