The introduction of long-acting PrEP represents a turning point in the global HIV response, especially for key populations who have historically faced persistent barriers to HIV prevention. Despite oral PrEP being introduced more than a decade ago, global uptake remains well below targets. New HIV infections are declining in some regions but are stagnating or rising in others, particularly where key populations are
underserved.
The Global Need for Long-Acting PrEP Among Key Populations
PxWire Volume 15, Issue 3
With the recent FDA approval of injectable lenacapavir (LEN) for PrEP and the drastic withdrawal of US investment in HIV prevention, the field must reimagine and recommit to getting PrEP rollout right this time AND to sustaining the HIV research pipeline. Research on HIV has brought numerous advances to global health, but controlling, and ultimately ending, the epidemic depends on continued investment in innovation.
This issue of PxWire looks at the scale of shuttered prevention programs for key populations (KPs), the potential market for injectable LEN, and the devastating cuts to research for an HIV vaccine.
Read below or download the PDF version of this issue.
Progress in PrEP Uptake
The PEPFAR stop work orders issued by the US government in January 2025 have devastated the HIV response worldwide, including funding for primary prevention. Sustaining the HIV response and bending the curve of incidence depends on identifying new sources of funding to maintain HIV prevention programs for KPs.
- Guidance issued in February 2025 indicated that PrEP services funded by the US government are permitted only for pregnant and lactating people—meaning that KPs, such as LGBTQ+ individuals, sex workers, and people who use drugs, have lost access to PrEP, unless they are currently pregnant or lactating.
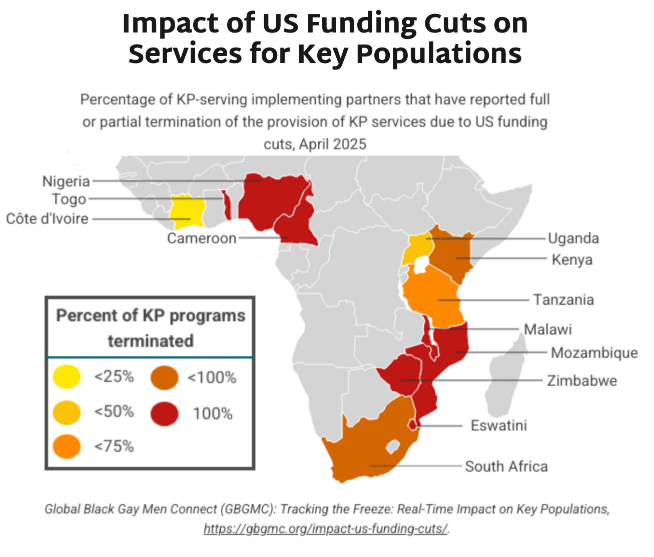
- The graphic below shows key findings on the percentage of KP programs terminated by country. Most of the priority African countries for the HIV response report national KP programs are fully or partially terminated.
- HIV incidence rates amongst KPs are higher than in other populations. Excluding this group from PrEP programming endangers whole communities threatened by HIV, disables the HIV response, and jeopardizes gains made against the epidemic.
- Research undertaken by Global Black Gay Men Connect (GBGMC) examines the impact of US government funding cuts on KP programs in Africa.
- Additional research done by AVAC, in the report Impact of PEPFAR Stop Work Orders, shows that KPs are the group most impacted by US government funding reductions to HIV prevention services worldwide. In some cases, such as Panama, national governments are stepping in to fill the gap.
PrEParing for New Products
Stakeholders—including Global Fund, PEPFAR, WHO, UNAIDS, Unitaid, Ministries of Health, advocates and implementing partners—have critical work to do now to ensure doses of LEN hit the ground as quickly as possible. Check out Gears of Lenacapavir for PrEP Rollout and Getting PrEP Rollout Right This Time to get the details.
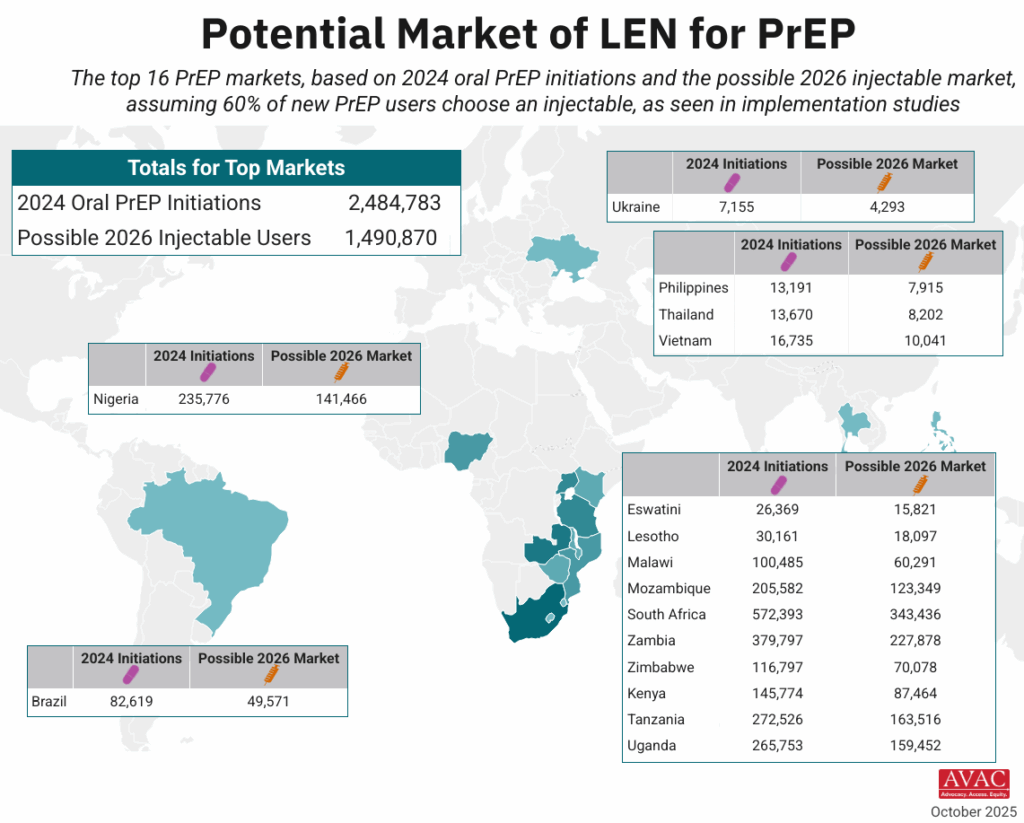
- The map shows 16 countries in Africa, Asia and Latin America that have the largest PrEP markets.
- If total PrEP initiations continue to increase by 20% every year, which is the trend in recent years, and injectables represent 60% of initiations, as seen in implementation studies, 2026 numbers of injectable initiations could be as shown in the map.
- The exact price and volumes of LEN per country is not yet known.
- Of those injectable initiations, LEN is expected to be the majority, given the proposed manufacturing projections from Gilead and the stated ambition of the Global Fund to reach two million people with LEN within the first three years of introduction. Additional volumes of injectable cabotegravir would make up the rest.
- PEPFAR, which in December committed to collaborate with Global Fund, has not yet publicly stated how LEN will fit into their more limited approach to PrEP, which has been restricted to pregnant and breastfeeding women.
The Latest R&D in the Prevention Pipeline
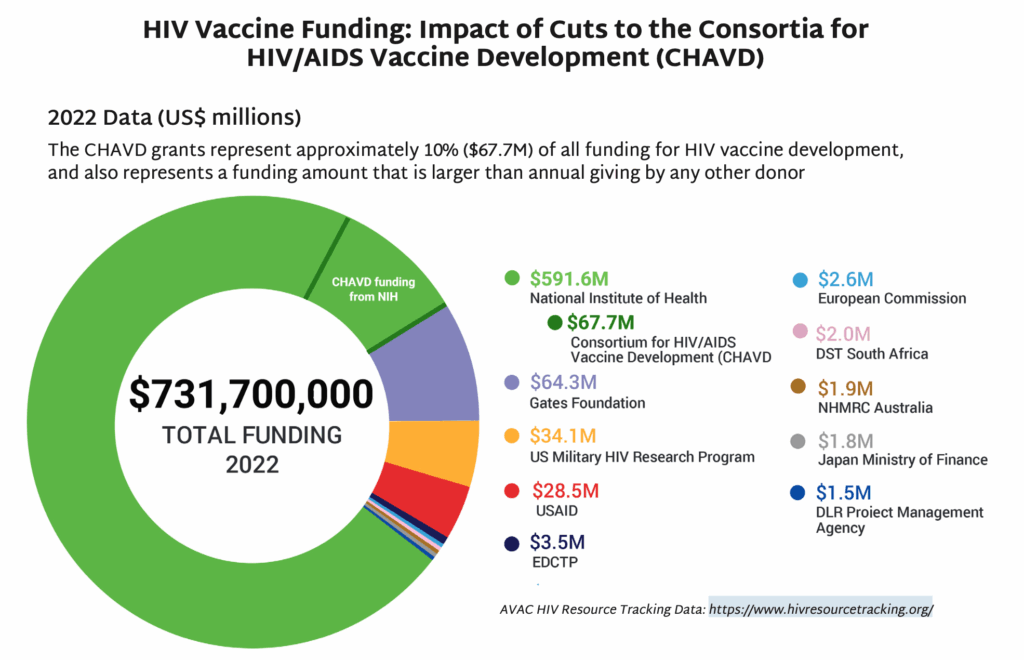
- In May, NIH’s National Institute of Allergy and Infectious Diseases (NIAID) announced that funding for the Consortia for HIV/AIDS Vaccine Development (CHAVD) would end after the current grant cycle in June 2026. With only one more year of funding before the grants end, current plans for research, clinical trials and progress toward a vaccine are all at risk.
- First launched in 2005, the CHAVDs led ground-breaking research to develop an HIV vaccine.
- CHAVD grants currently fund two institutions as consortia leaders—The Scripps Research Institute and Duke University.
- The quest for an HIV vaccine is gaining momentum with field-changing contributions from the CHAVDs. The institutions are currently researching vaccine designs that rely on the immune system’s broadly neutralizing antibodies (bNAbs) to protect against HIV.
- The annual funding for the consortia—approximately $67M—represents a significant chunk of the NIH’s funding for HIV vaccine development, and also approximately 10% of all funding for HIV vaccine research globally each year.
- NIH’s yearly grant total for the CHAVD is larger than any other individual donor’s annual giving for HIV vaccine research. The next closest donor is The Gates Foundation, which donates approximately $64M a year to this research.
- In 20 years of research, CHAVD discoveries have resulted in new technology to combat HIV, influenza, Zika, COVID, and other novel coronaviruses. The loss of the CHAVD will have a devastating impact on the HIV response and scientific discovery.
Prevention Playlist
AVAC develops a wide range of resources to inform decision making and action. Check out the latest:
Join
- CHANGE: In response to the unfolding crisis, more than 1,500 people from civil society organizations around the world have launched CHANGE—Community Health & HIV Advocate Navigating Global Emergencies—a coalition formed to support urgent action: [email protected]
- Subscribe to Global Health Watch: AVAC’s weekly newsletter dedicated to breaking down critical developments in US policies and their impact on global health, at avac.org/global-health-watch
- Fight For Our Lives” Emergency Townhall: Impact of the Trump Administration Foreign Aid Freeze on KP & LGBTQ Communities, Ongoing convening, Register here
Use
- Advocacy Resources for Injectable LEN, AVAC
- HIV Prevention R&D at Risk, AVAC
- Impact of PEPFAR Stop Work Orders, AVAC
- Research Matters Advocacy Toolkit, AVAC, TAG, HIVMA
- Advocates’ Guide: Understanding the President’s Proposed Fiscal Year 2026 (FY26) Budget and Its Implications for Science, Research and Global Health, AVAC
Watch/Listen
- FDA Approves Injectable LEN for PrEP
- Fight for Firewalls: HIV and Health Data Privacy in the Snowballing Surveillance State, Webinar
- Embracing Task Shifting and Innovation to Support Expanded Access to Long-Acting Injectable PrEP, Webinar
- The Scientific Journey of Lenacapavir: From basic science to clinical development to impact, Webinar
- Critical Advocacy: How Civil Society is defending the HIV Response and Global Health, Podcast
- HIV Prevention at a Crossroads: Why we still need an HIV vaccine, Webinar
- A New Era of HIV Prevention: Accelerating access to long-acting prevention options through sustainable prevention systems and financing, Webinar
Read
- FDA Approves Injectable Lenacapavir for PrEP, AVAC
- Trump Aid Cuts Deal a Blow to HIV Prevention in Africa, Reuters
- Will long-lasting HIV preventive be a game changer—or a missed opportunity?, Science
- Regulators Approve a Twice-Yearly Shot to Prevent HIV Infection, New York Times
- FDA Approves Twice-Yearly Lenacapavir for HIV Prevention, POZ
- FDA approves twice-yearly shot for HIV prevention, Helio
- BREAKING: FDA approves breakthrough drug that reduces risk of contracting HIV by 96 percent, The Advocate
- Getting PrEP Rollout Right This Time: Lessons from the field, AVAC
- Why STI Funding Matters, AVAC
- AVAC Condemns Removal of the Advisory Committee on Immunization Practices, AVAC
- AVAC Denounces White House Effort to Codify DOGE Cuts to Health, Research and Foreign Assistance, AVAC
- Protect Federal Funding for HIV, TB, and STI Research and Prevention at the National Institutes of Health, AVAC
- The People’s Research Agenda, AVAC
- Worldwide Prevention, Shared Protection: Why STI Funding Matters, AVAC
Long-Acting PrEP Market Assessment for Key Populations
This market assessment supports countries, donors, implementing partners, and advocates in making informed decisions about the introduction, scale-up, and equitable delivery of long-acting PrEP among key populations and other priority groups.
Getting PrEP Rollout Right This Time
Thirteen years after oral PrEP’s introduction, global uptake remains slow, with just over 9 million initiations—falling short of UNAIDS’ 2025 targets. While HIV infections are declining in some regions, others are experiencing increasing epidemics. Despite challenges, lessons from oral PrEP and CAB rollout have strengthened health systems for scaling up longer-acting PrEP.
AVAC undertook a qualitative landscaping analysis to identify actionable lessons and recommendations across seven countries—Brazil, Kenya, Nigeria, South Africa, Vietnam, Zambia, and Zimbabwe—exploring themes of:
- Successes and challenges with daily oral PrEP introduction and scale-up and what can be done differently for new PrEP products.
- Public health system readiness for the introduction and scale-up of new PrEP products.
- Considerations for improving and accelerating PrEP regulatory approval, normative guidance, demand generation, stakeholder engagement, and health systems strengthening.
This analysis, conducted across seven countries before the January 2025 US foreign aid freeze, identifies urgent actions to sustain momentum. US foreign aid cuts are severely impairing PrEP delivery, jeopardizing remarkable progress made in the last 20 years. In the wake of this disruption, it is vital that efforts to mobilize and sustain an effective HIV response learn from the past and reach UNAIDS’ targets for 2030 by incorporating these insights into future planning.
Visit PrEPWatch for the full report and individual country profiles.
Advocates’ Guide: Understanding the President’s Proposed Fiscal Year 2026 (FY26) Budget and Its Implications for Science, Research and Global Health
The US administration’s proposed Fiscal Year 2026 (FY26) budget marks a sweeping rollback of federal investment in health, research, and global development. For advocates, researchers, and implementers, this proposal demands urgent attention and action.
This initial “skinny budget” is a proposal and not yet law. A more detailed proposal will be released by mid-to-late May and the US Congress will ultimately decide actual funding levels for FY26, which begins October 1. So, advocates must speak up now to protect funding for research and programming that saves lives and livelihoods.
Here’s what advocates need to know and do:
Big Picture: A Dramatic Retrenchment
The budget proposes $163 billion in cuts to non-defense discretionary spending, including a 26% reduction to the Department of Health and Human Services (HHS)— the department that oversees the US National Institutes of Health (NIH), the Centers for Disease Control and Prevention (CDC), and the Food and Drug Administration (FDA). These cuts are completely offset by an increase to defense spending and reflect a shift toward the elimination of science and programming tied to diversity, equity, inclusion (DEI), gender, and climate, and a redirection of funding toward defense and “America First” priorities—priorities that put the perceived interests of the US and its citizens over other national and global issues.
Detailed Analysis and Implications
Health and Biomedical Research
The proposed cuts to HHS would gut federal support for health and biomedical research, dismantling key programs at NIH and CDC. They threaten progress on infectious diseases, health equity, and pandemic preparedness—undermining decades of scientific gains and leaving communities vulnerable.
NIH is Cut by $17.9 billion losing HIV and global health research
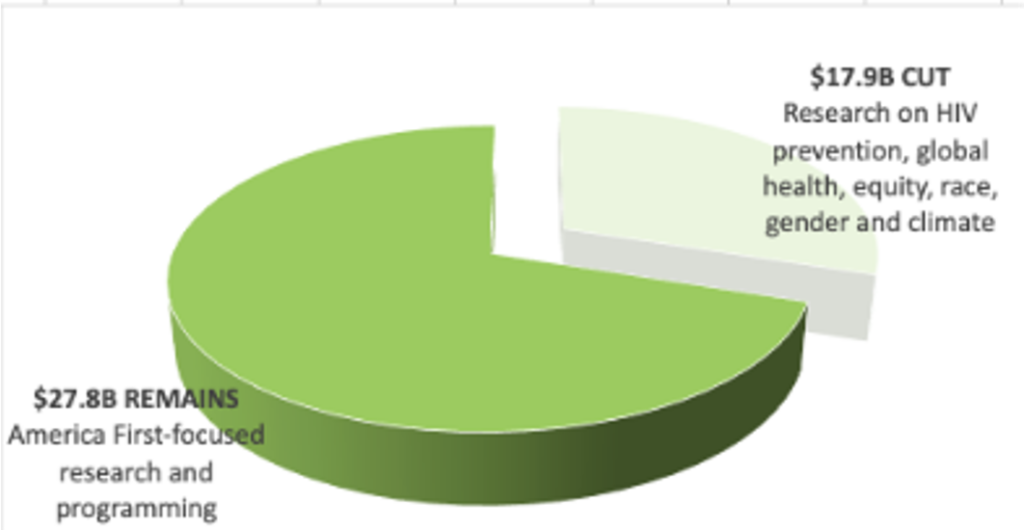
- Preserves $28 billion of the $46 billion for NIH overall, but excludes HIV prevention, global health, and health equity research.
- Reorganizes NIH into 5 “realigned” institutes, removing focus on climate, gender, racial equity.
- Eliminates the Fogarty International Center and the National Institute on Minority Health and Health Disparities.
Centers for Disease Control and Prevention (CDC): Cut by $3.59 billion
- Eliminates Global Health Center and National Centers on environmental health, injury prevention and chronic disease prevention.
- Eliminates DEI programs and shifts the burden for pandemic prevention and response.
Agency for Healthcare Research and Quality: Effectively eliminated
- Cited as redundant; targeted for work on climate and gender.
National Science Foundation (NSF): Cut by $4.9 billion (56%)
- Eliminates funding for work seen as ideologically objectionable (e.g., broadening participation and racial equity in STEM).
Global Health and Development
At a time when the USG should be expanding access to new technologies, the proposed FY26 budget guts foreign assistance funding, threatening pillars of the global HIV response: the President’s Emergency Plan for AIDS Relief (PEPFAR) and US contributions to multilateral initiatives, such as Global Fund and GAVI. The ideological targeting of family planning and gender-related programs will further weaken interventions to address HIV, which have been shown to work best within a comprehensive package of health and social services.
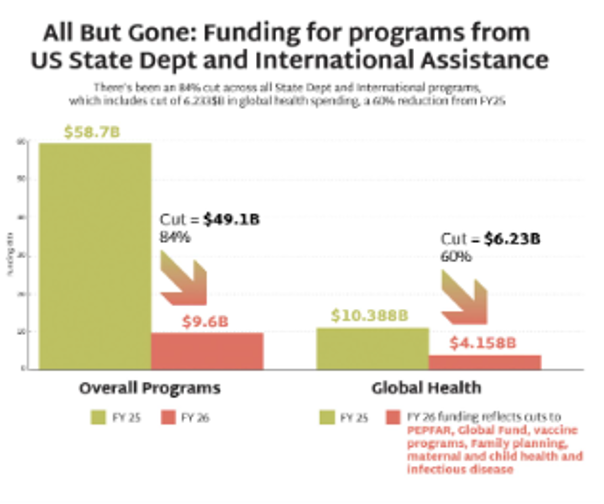
Global Health Programs: Cut by $6.23 billion
- Defunds NGOs providing family planning, impacting maternal and child health providers.
- PEPFAR preserved only for existing treatment programs and programs for the prevention of mother-to-child transmission (PMCT) , and specifically excludes primary prevention and PrEP, except for pregnant and lactating populations.
USAID Development Aid: Cut by $8.33 billion
- USAID is eliminated with the limited number of existing programs moved into the State Department.
- Eliminates DEI, climate, and gender-related programming.
- Creates new “America First Opportunity Fund” to replace foreign assistance grants with loans that prioritize US interests over humanitarian needs.
Centers for Disease Control and Prevention (CDC)
- STI, TB, hepatitis programs folded into a reduced $300 million block grant.
- For more on the specific impacts on STI programs, please read AVAC’s new brief on Worldwide Prevention, Shared Protection: Why STI Funding Matters.
Health Resources and Service Administrations (HRSA): Cut by $1.73 billion
- Ryan White HIV/AIDS Program activities not deemed core are eliminated.
Substance Abuse and Mental Health Services Administration (SAMSHA): Cut by $1.065 billion
- Eliminates harm reduction and regional substance use program grants.
Offices of Minority & Women’s Health
- Moved under a new, less visible structure.
New Initiative: “Make America Healthy Again”
- $500 million focused on lifestyle over treatment.
What This Means
- HIV Prevention R&D and global implementation is at risk. Cuts to NIH and USAID directly threaten support for clinical trials, community engagement, and biomedical innovation.
- Equity-centered research threatened. Eliminating institutes focused on minority and global health severely undermines inclusive science and jeopardizes future impact. Inclusion is not just a nice to have, it’s integral to achieving impact
- PEPFAR protections are narrow. Only existing beneficiaries are covered; scale up and innovation are excluded, compromising the imminent introduction and potential impact of injectable lenacapavir for PrEP. Funding for HIV prevention is also eliminated, except for pregnant and lactating populations.
Advocacy Priorities
- Monitor the full FY26 budget release for agency-level detail and justification.
- Engage Appropriations and other relevant Committees via coalition efforts (e.g., FAPP, GAPP, GHTC, SHF).
- Mobilize your community to contact your Senators and Representatives to let them know you oppose these cuts.
- Share your stories from researchers affected by cuts—particularly those whose work is globally focused or funded by NIH/USAID.
- Stay up to date with budget briefings and mobilization opportunities. See AVAC’s ‘Research Matters’ resource, which shares guidance and a toolkit for researchers to advocate for continued funding.
This budget is a threat to decades of progress in science, equity, and health—but it is also an opportunity to speak with clarity and urgency about what is at stake. Advocates must ensure that the future of HIV prevention, global health innovation, and equitable science is not written by politics, but by people, evidence, and impact.
Worldwide Prevention, Shared Protection
This Issue Brief describes the impacts of the elimination and reduction of funding that supports sexually transmitted infection (STI) research, testing, and prevention programming. This funding is critically important as STI rates continue to increase globally with more than 1 million curable STIs, including chlamydia, gonorrhea, syphilis, and trichomoniasis, acquired every day. Without appropriate testing, treatment, and prevention programs, there is a risk that STI rates will continue to increase leading to more cases of infertility, pelvic inflammatory disease, and cancers. Further, there is the risk that we will lose the ability to monitor, react to, and prevent the rise of antimicrobial resistant gonorrhea, which has become an issue for the majority of treatment options currently available.
PxWire Volume 15, Issue 2
The field of HIV prevention is confronted with two opposing forces; programs for delivering PrEP have been shuttered all over the world by the withdrawal of the US government from global health. At this same moment in history, next-generation long-acting products hold great promise to accelerate HIV prevention and help the world achieve epidemic control. Navigating these seismic developments requires unprecedented coordination, solidarity, and courage.
Global health champions can defy the hatred, fear, and greed that are dominating politics in so many places around the world. Together we can innovate, create, and protect the advance of HIV prevention and global health. This issue provides a snapshot on threats to delivering PrEP, the potential of injectable lenacapavir (LEN) for PrEP, and on the implications of upstream research and development of other long-acting PrEP.
Read below or download the PDF version.
Progress in PrEP Uptake: Threatened
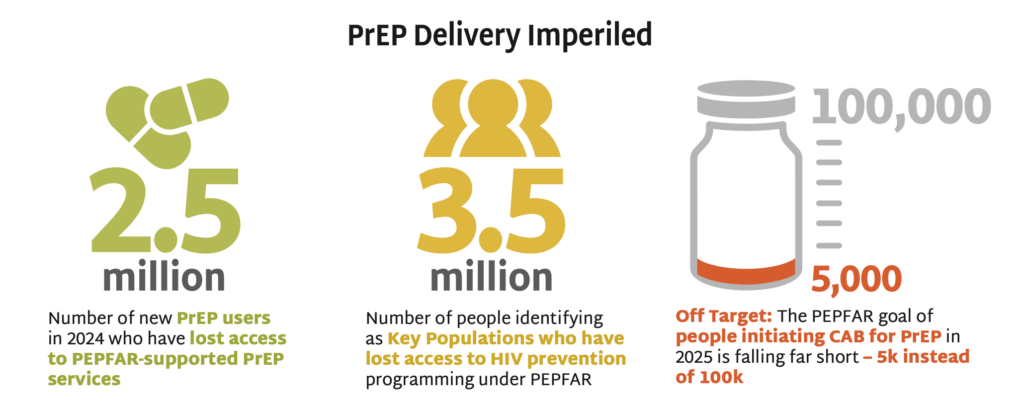
- PEPFAR documented 2.5 million new PrEP users in 2024, who could now lose access to PEPFAR- supported PrEP services. US Department of State issued a limited and inconsistently implemented waiver in February, allowing for continued provision of HIV treatment but restricting PrEP access to pregnant and lactating people only.
- These actions will result in 3.5 million who identify as key populations (KPs) losing access to all HIV prevention programming under PEPFAR, according to 2024 PEPFAR data tracking KP use of PrEP. These groups have higher rates of HIV incidence and face additional barriers to accessing services now that targeted programs are suspended.
- PEPFAR had a goal of 100,000 people initiating cabotegravir (CAB) for PrEP in 2025. But only 5,000 individuals had initiated by October 2024. The suspension of PEPFAR funding imperils scale-up of this long-acting product.
- These figures represent just some of the disruptions that are decimating PrEP delivery. Learn more here: Impact of PEPFAR Stop Work Orders on PrEP
- Overcoming this challenge, restoring, sustaining, and accelerating PrEP access is imperative and possible if the field works together.

For the last eight years, AVAC has proudly worked with PEPFAR to document PrEP uptake and its impact around the world. That stopped in January with a stop work order from the US government. But protecting access to PrEP is vital. Are you leading a PrEP program? Whether supported by PEPFAR or not, we invite you to work with us to ensure global data on PrEP is not lost. Find us at [email protected].
PrEParing for New Products
- Approval by the US Food and Drug Administration (FDA) for injectable 6-month LEN for PrEP is expected in June, with WHO guidelines expected in July. See the full timeline.
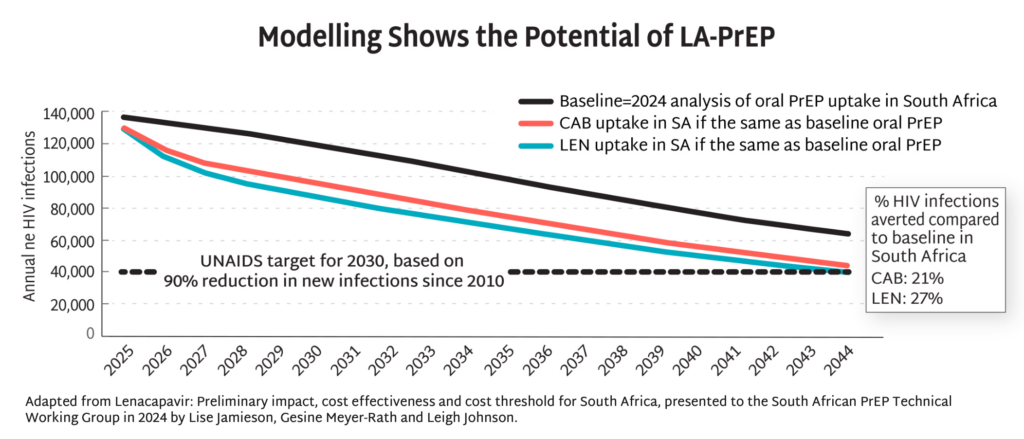
- Modelling data from South Africa demonstrate the potential of injectable PrEP to dramatically reduce HIV incidence by up to 90% by 2044, and potentially even sooner with more aggressive uptake. This potential goes beyond South Africa, lighting the way toward epidemic control the world over.
- The field must be prepared for swift action once LEN is approved and recommended, to ensure this opportunity is not squandered. As AVAC’s interactive timeline, Tracking LEN Rollout, outlines, donors, ministries of health, manufacturers, regulators, and civil society all have a role to play to pave the way for swift, equitable and effective introduction of LEN for PrEP.
The Latest R&D in the Prevention Pipeline
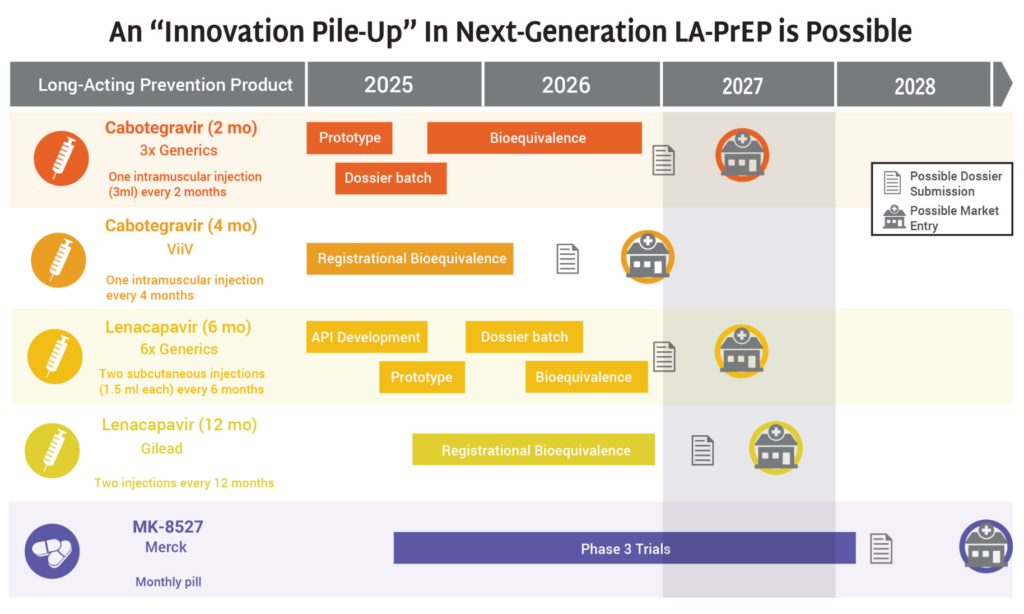
- The HIV prevention market is headed toward a period of significant opportunity—and possible congestion—as a slate of new products are on track for continued development and potential introduction to the market in 2027 and 2028.
- Generics for 2-month CAB and 6-month LEN, along with ViiV’s 4-month CAB, Gilead’s 12-month LEN, and Merck’s monthly oral MK-8527 PrEP pill (if further development and approvals are successful) could all enter the market by 2028.
- The possibility of so many products on the market, including four different formulations of injectable PrEP, means that it is imperative the field prepares for this future now.
- Markets and policies must be built to support the products in the market already, so that new options can be rapidly deployed and deliver impact. Otherwise, the field will squander time and money, with epidemic control slipping further out of reach.
- With US funding cuts to both HIV prevention R&D and delivery, communities must be engaged, supported, and informed about all prevention options, and the choices that all stakeholders will need to make. This means gathering and sharing data and information about cost-effectiveness, user acceptability, program feasibility, and impact. Communities empowered with the facts can advocate for the choices they need, and push ministries of health to make strategic investments and procure the prevention method mix that fits their context and delivers impact.
Prevention Playlist
AVAC develops a wide range of resources to inform decision making and action. Check out the latest:
Join
- Subscribe to Global Health Watch: AVAC’s weekly newsletter dedicated to breaking down critical developments in US policies and their impact on global health, at avac.org/global-health-watch
- CHANGE: In response to the unfolding crisis, more than 1,500 people from civil society organizations around the world have launched CHANGE—Community Health & HIV Advocate Navigating Global Emergencies—a coalition formed to support urgent action: [email protected]
Use
- Research Matters Advocacy Toolkit, AVAC
- Tracking the Freeze: Real-Time Impact on Key Populations, GBGMC
- Impact of the Stop-Work Order on PrEP, AVAC
- Tracking Lenacapavir Rollout, AVAC
- PEPFAR Program Impact Tracker, Impact Counter
- Weekly Situation Report, UNAIDS
Watch/Listen
- Politics and Global Health: The Need for a New, Resilient Architecture, Webinar
- Lawsuit Wins and What’s at Stake: AVAC v US Department of State, PxPulse episode
- Global Health in the Lurch: What’s happening now and who is pushing back, PxPulse episode
- Advancing Sexual and Reproductive Health and Rights: Moving forward post-the 2024 US Election, Webinar
Read
- Despite USG Global Health Collapse, Here Are Several Data Trackers To Support Your Advocacy, AVAC
- On Going Backwards, Lancet
- The Trump Administration’s Foreign Aid Review: Status of PEPFAR, KFF
- The Best Investment You Didn’t Know You Made: How NIH Funding Fuels Innovation and Economic Growth, amfAR
- HIV Market Impact Memo, CHAI
- The USAID List of Terminated Global Health Awards – What Does it Tell Us?, KFF
- AVAC Condemns HHS Mass Layoffs
- PrEP in the Balance: Hopes and fears in 2025
- Better Engagement, Better Evidence: Working in partnership with patients, the public, and communities in clinical trials with involvement in good participatory practice, Lancet
- PEPFAR: A Strategic Necessity for US Leadership and Global Health, AVAC
- Rallying for HIV Prevention Amid Policy Attacks, AVAC
- CROI 2025 Shows the Promise of Research at its Best, AVAC
PxWire Volume 15, Issue No. 1
In this special edition of Px Wire, AVAC is going beyond a quarterly update of biomedical HIV prevention. In this issue, we look at how the new US Administration’s attack on global health can be expected to devastate HIV prevention, including the capacity to deliver existing PrEP options, the scale up of new PrEP products, and the paralyzing impact on research and development. A PDF version of this report is also available.
From Research to Rollout: The impact of USG global health pullout


The United States’ presidential regime has launched a sustained, multi-pronged attack against foreign assistance, scientific inquiry, due process and good governance. It threatens economies, human rights, international partnerships, global health at large, and the rule of law. For HIV prevention, a single sentence, issued in a February 6 advisory from the US Department of State, has derailed the entire field, potentially setting back the HIV response by years, if not decades. Read on for resources to support your advocacy and fortify our solidarity at this critical time.
Progress in PrEP Uptake: Threatened
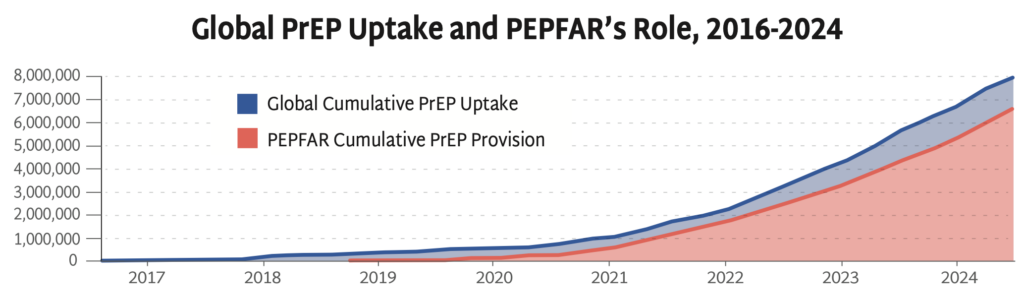
PEPFAR has been pivotal to accelerating PrEP uptake, significantly expanding HIV prevention coverage. The freeze on foreign aid prohibits funding to PEPFAR’s PrEP programs and poses a serious threat to global efforts to control the epidemic.
AVAC’s Global PrEP Tracker has documented cumulative PrEP initiations on a quarterly basis for nearly a decade. This graph presents the final data collected while PEPFAR was fully operational—PEPFAR support was responsible for 79% of PrEP uptake globally in the last year and reached 83% by the end of September of 2024. Data on the fourth quarter of 2024 is inaccessible since PEPFAR was taken offline in late January.
At the time of the foreign aid freeze, PrEP uptake had reached 8 million initiations since 2016, an achievement that’s taken almost 10 years to reach—too slow and too small to reach UNAIDS targets, but a robust foundation to finally accelerate PrEP uptake with next-generation PrEP. Current US policies, instead of expanding PrEP coverage, are leading to the closure of programs, and will reverse global progress against HIV.
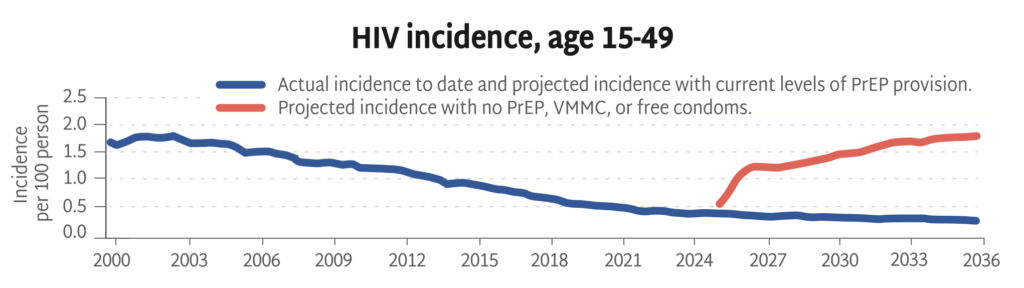
Without primary prevention, the HIV epidemic is poised to rage on, with incidence among adults on track to triple over the next ten years. This HIV Synthesis model, developed by the HIV Modelling Consortium, estimates the impact of stopping all HIV prevention services across Africa from now through 2036—including PrEP, voluntary medical male circumcision (VMMC), and free condom distribution.

For the last 8 years, AVAC has proudly worked with PEPFAR to document PrEP uptake and its impact around the world. That stopped in January with a stop work order from the US government. But protecting access to PrEP is vital. Are you leading a PrEP program? Whether supported by PEPFAR or not, we invite you to work with us to ensure global data on PrEP is not lost. Find us at [email protected].
PrEParing for New Products: Is rollout still possible?

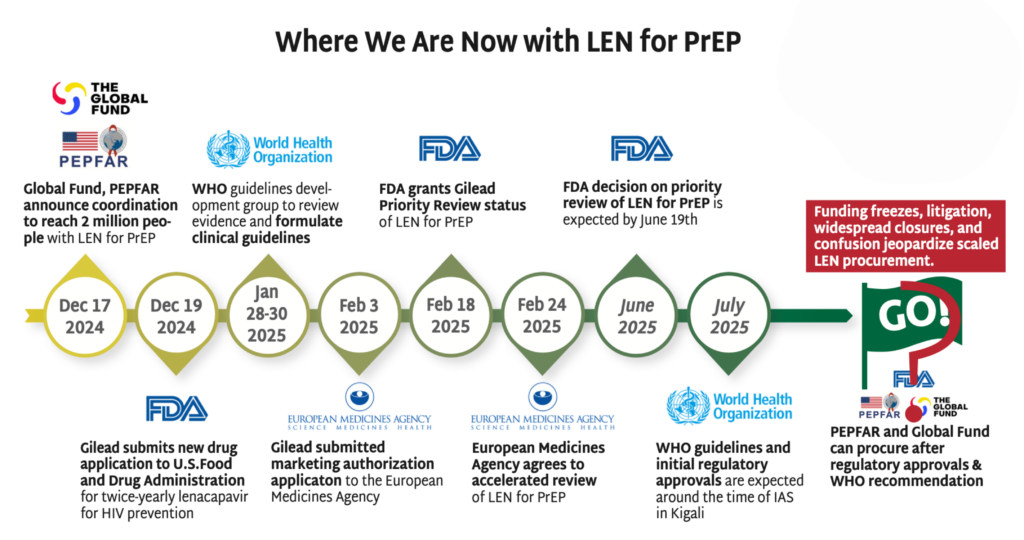
Read more in The Gears of Lenacapavir for PrEP Rollout.
The chaos in foreign assistance programs (including discontinuation of major PrEP programs), cuts in staffing and new demands on donor commitments will make decisions on the procurement of LEN for PrEP more complex and uncertain. In December 2024, the Global Fund and PEPFAR announced a plan to reach 2 million people with LEN for PrEP over three years. Exactly how funding to support this unprecedented introduction program will move forward, in the absence of significant US investment, is far from certain. The other stakeholders, including Global Fund, Gilead, CIFF and the Gates Foundation expressed commitments to the deal, but major questions remain. In the meantime:
- Gilead’s production of LEN for PrEP is continuing, as is the technology transfer to generic license holders.
- The FDA granted Gilead priority review status for LEN for PrEP, with a decision due by June 19, 2025.
- The EMA has agreed to an accelerated review of LEN for PrEP for both European access and as part of the EU-Medicines for all (EU-M4all) program, reducing the review from seven months to five months.
- The WHO’s Guideline Development Group (GDG) met in January, and WHO is expected to issue guidelines by July 2025.
The Latest R&D in the Prevention Pipeline: Supported or undermined?

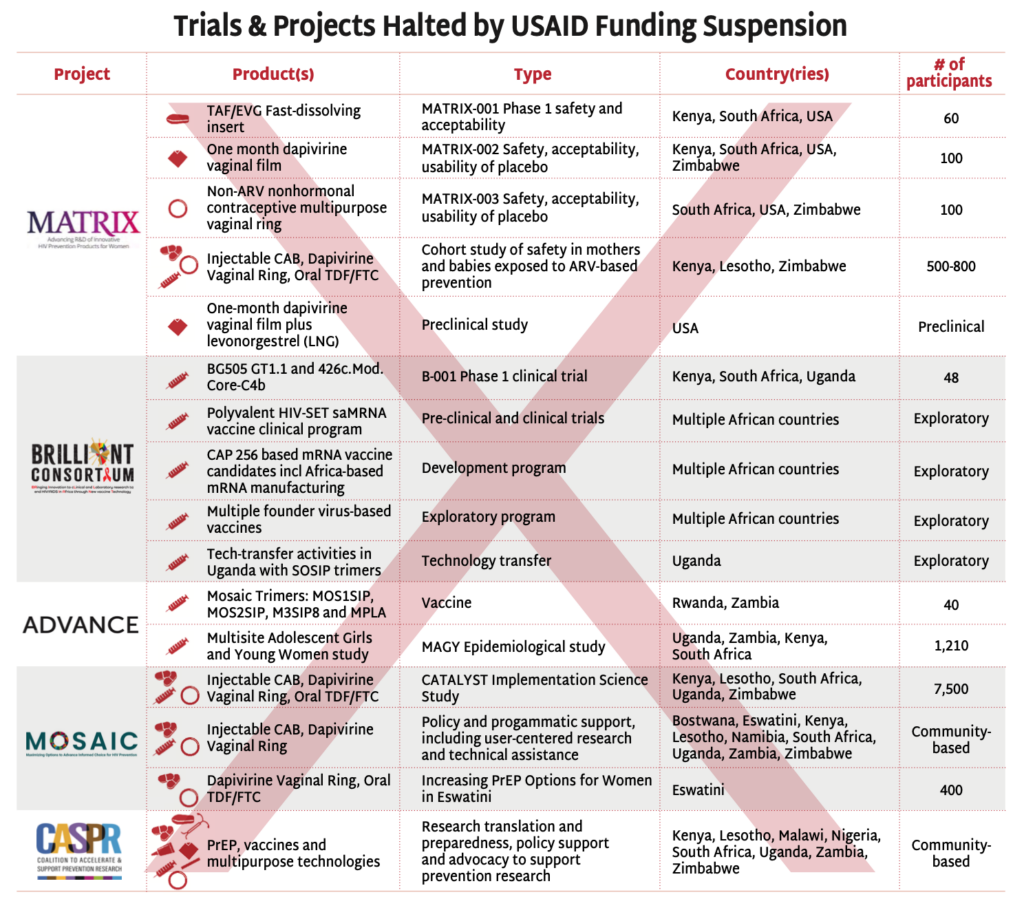
The stop-work orders have disrupted USAID-supported HIV prevention research, halting critical investigations in vaccine and next-generation PrEP strategies.
- The BRILLIANT and ADVANCE projects’ clinical, preclinical, and experimental trials testing HIV vaccine candidates have been suspended.
- The MATRIX projects’ driving innovation with next-generation PrEP and MPT products, fast-dissolving inserts and vaginal films and rings, have been forced to stop their clinical trials.
- The MOSAIC projects’ have suspended all implementation science activities, including the CATALYST study, investigating choice among oral PrEP, injectable cabotegravir and the dapivirine vaginal ring. Other implementation studies are continuing, but access to the commodities, much of which was procured by PEPFAR is questionable. See AVAC’s Integrated Study Dashboard for details.
- The Coalition to Accelerate and Support Prevention Research (CASPR) has also been paused. Led by AVAC in partnership with a number of leading African civil society organization, CASPR focuses on building an enabling environment for HIV prevention R&D. (Note: In early February, AVAC lead a lawsuit against the State Department seeking emergency relief from the freeze on foreign assistance, including funding for CASPR. The case, AVAC v. United States Department of State, is pending.)
These disruptions delay the development of urgently needed HIV interventions and threaten the sustainability of research infrastructure all over the world, with particularly egregious impacts on the research capacity of regions most impacted by the epidemic.
The abrupt suspension of these trials also raises serious ethical concerns. Stopping trials mid-course undermines trust in research, jeopardizes community engagement, and abandons participants who volunteer their bodies for scientific discovery. It will take years to build back this critical infrastructure—for HIV research and beyond—as well as the community partnership and trust needed to ensure smooth and ethical research.
Prevention Playlist
AVAC develops a wide range of resources to inform decision making and action. Check out the latest:
Join
- Subscribe to Global Health Watch: AVAC’s weekly newsletter dedicated to breaking down critical developments in US policies and their impact on global health. avac.org/signup
- Seeking Visuals and Videos: Leading groups in Washington, DC, are urgently trying to collect videos and photos documenting the impact of the US government’s foreign aid freeze, such as clinic closures despite the waiver. Non-professional phone videos and photos are welcome. Send to [email protected] for more details
- CHANGE: In response to the unfolding crisis, more than 1,300 people from civil society organizations around the world have launched CHANGE—Community Health & HIV Advocate Navigating Global Emergencies—a coalition formed to support urgent action. [email protected]
Use
- Graphic of studies of injectable cabotegravir and the dapivirine vaginal ring in eastern and southern Africa, AVAC
- Most Lifesaving Services Remain Paused: A Rapid Assessment of the PEPFAR Stop Work Order, amfAR, CHANGE, Data ETC
- What Effect are HIV Programmes Having in Africa, The HIV Modelling Consortium
- PEPFAR & Global Fund Support for HIV Programs, amfAR & Data ETC
Watch/Listen
- [LISTEN] Would PEPFAR Survive Trump—and what would it look like?
- The Impact and Implications of Recent US Government Federal Funding Reductions on Health Programmes, The Steve Biko Centre for Bioethics at The University of the Witwatersrand event recording
Read
- AVAC v United States Department of State. On February 10, 2025, AVAC and another nonprofit organization sued the new US Administration, seeking emergency relief from an Executive Order that inhumanely froze all funding for foreign assistance, AVAC
Gears of Lenacapavir for PrEP Rollout
The Gears of Lenacapavir for PrEP Rollout outlines a transformative opportunity in the global fight against HIV, coming at a pivotal moment when the scale-up of PrEP has shown remarkable progress but remains insufficient to achieve a transformational impact on HIV incidence and the trajectory of the epidemic. This effort demands a coordinated response from governments, donors, civil society, and the private sector to ensure rapid implementation, equitable access, and sustainable impact. By leveraging lessons from previous PrEP interventions, aligning financing mechanisms, and prioritizing underrepresented regions, stakeholders can overcome systemic barriers and maximize the public health potential of this innovative long-acting prevention tool.
With anticipated regulatory approvals and production scaling, the plan targets over 2.5 million LEN users in low- and middle-income countries by 2027. With a focus on addressing structural barriers like stigma, healthcare inequities, and restrictive policies, alongside integrating generics into national programs, the roadmap aims to build on existing progress while accelerating the pace of HIV prevention.
AVAC’s Most Downloaded Resources of 2024
From the implementation of DoxyPEP to the game-changing trial results of lenacapavir for PrEP, 2024 has been a landmark year for advancements in HIV and STI prevention. AVAC’s most downloaded resources capture these pivotal milestones, offering essential insights and tools to power your advocacy. Dive into the highlights and stay informed about the strategies shaping the future of HIV prevention.
AVAC’s Top 10

This episode of PxPulse looks at why and how the decisions that shape global health must be made by those facing the greatest risks. As the world evaluates the pandemic response and debates on decolonizing global health gain momentum, equity in global health has never been more urgent.

This graphic shows currently available options for HIV prevention, newly approved and recommended treatment, and those in development.

This plan provides a broad view of all the moving parts and identifies actions and actors responsible for ensuring time is not wasted and opportunity not squandered.

This PxPulse podcast episode goes deep on LEN for PrEP. Recorded just days before Gilead’s announcement that PURPOSE 2 also found very high efficacy, Dr. Flavia Kiweewa, a principal investigator of the first trial to announce efficacy, lays out the research findings and what they mean. And Chilufya Kasanda Hampongo of Zambia’s Treatment Advocacy and Literacy Campaign and Mitchell Warren of AVAC talk about how to change a long history of squandered opportunities to get rollout right.

This report examines disbursements by the U.S. NIH and the Bill & Melinda Gates Foundation and is one of few reports to track funding trends in vaccine and diagnostics R&D, and pipeline investments for some of the most common STIs.

Led by AVAC alongside a network of partners, the People’s Research Agenda puts forward recommendations to diversify and strengthen the HIV prevention pipeline, enhance investment and financial support for HIV prevention research and development, and guide an advocacy strategy that truly addresses the needs of communities across the prevention pipeline.

This roadmap aims to build on existing progress while accelerating the pace of HIV prevention. With anticipated regulatory approvals and production scaling, this plan targets over 2.5 million LEN users in low- and middle-income countries by 2027. It focuses on structural barriers and integration of generics into national programs.

Good Participatory Practice Guidelines have been shaping and improving clinical research since 2007. They provide a global reference guide for ethical and effective stakeholder engagement, helping ensure the priorities of trial participants and their communities are centered in clinical trials and broader research agendas.

