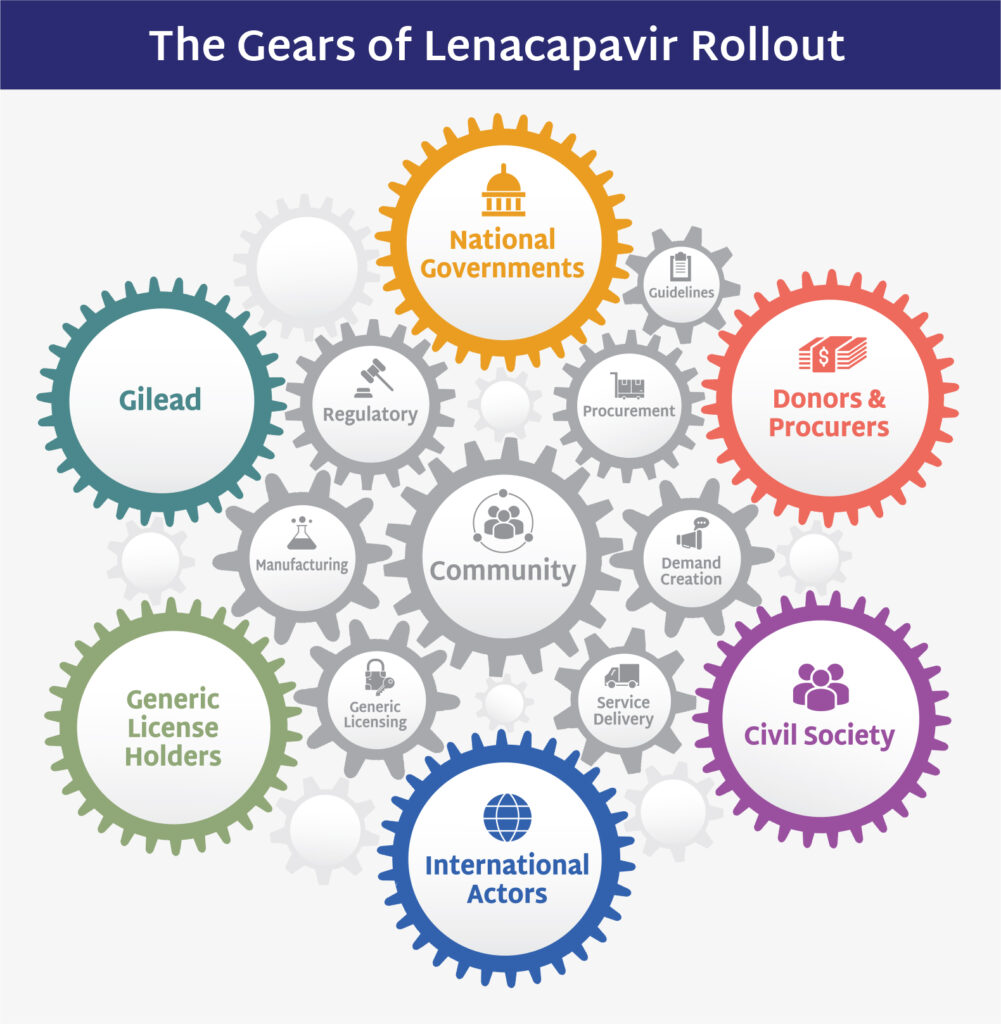
This week we saw Congress pass the President’s US domestic policy agenda that will rob approximately 17 million Americans of access to health care, including people living with and vulnerable to HIV, in order to provide tax cuts and workarounds for the rich. We also saw the official closure of USAID; restructuring of WHO leadership amid funding shortfalls; the US Supreme Court’s ruling preserving preventive care, including PrEP, and another ruling limiting federal courts’ ability to block presidential actions.
Read on for more, and be sure to check out the latest issue of PxWire that looks at the scale of shuttered prevention programs for key populations (KPs), the potential market for injectable lenacapavir, and the devastating cuts to research for an HIV vaccine.
Congress Passes Sweeping Bill to Fulfill President’s Domestic Agenda
Both chambers of the US Congress narrowly passed a massive legislative bill that now codifies, or makes legal, the Presidents domestic policy agenda that extends tax cuts to the wealthy at the expense of social safety net programs. These programs, like Medicaid, were slashed in a futile effort to soften the impact of the bill on dangerously growing the federal deficit.
IMPLICATIONS: The bill will lead to at least 51,000 excess deaths annually in the US in order to provide ultra-wealthy individuals with an average $309,000 in annual tax cuts. This “Big Bad Betrayal” of a bill not only cuts effective and cost-efficient health care for the poor and uninsured, but also food and nutritional assistance, housing, and adds billions to the growing budget deficit to target, detain, and deport immigrants nationwide.
READ:
- Cuts Kill: House Passes Deadly Reconciliation Bill Cutting Access to Healthcare & Decimating the Nation’s HIV Response—Save HIV Funding campaign release
- House Passes Sweeping Bill to Fulfill President’s Domestic Agenda—New York Times
- ‘Big, beautiful’ budget reconciliation package passes Senate—Roll Call
USAID Officially Closes
The US Agency for International Development (USAID) officially closed this week, marking the end of a transformative era in US global health and development leadership—the agency was first founded in 1961 as a mandate from President John F. Kennedy. Past US presidents, former agency employees, advocates and celebrities memorialized the occasion by noting the impact of the agency and its work over the decades. The closure comes amid significant and questionable downsizing and funding cuts initiated by the US DOGE Service, including a proposed rescissions package of $400 million in Congressionally approved PEPFAR funds, which shatters a bipartisan legacy that has delivered health, equity, and collaboration around the world. Remnants of USAID are now being folded into the US State Department.
IMPLICATIONS: The shuttering of USAID jeopardizes the progress made in global health and development, weakens US credibility as a global health leader, and creates a vacuum that could be filled by other superpowers. A study published this week in the Lancetestimated that USAID programs have saved 90+ million lives in two decades and that if the current cuts continue through 2030, 14 million people who might have otherwise lived could die. Specifically for HIV, at a time when scientific breakthroughs such as injectable lenacapavir for PrEP could accelerate HIV epidemic control, the dismantling of the global health infrastructure needed to deliver these tools threatens to squander this opportunity towards achieving sustainability.
READ:
- A Second Opinion—Former Republican Senator and Majority Leader Bill Frist’s Substack
- Farewell to USAID: Reflections on the agency that President Trump dismantled—NPR
- WATCH: How a new twice-yearly drug is prompting hopes of curbing HIV cases—PBS NewsHour
- Evaluating the impact of two decades of USAID interventions and projecting the effects of defunding on mortality up to 2030: a retrospective impact evaluation and forecasting analysis—The Lancet
- Study: 14 million lives could be lost due to Trump aid cuts—NPR Goats & Soda
- U.S.A.I.D. Might Be Dead, but the Waste Is Alive and Well—New York Times
- Bono Bids Emotional Farewell to USAID Staffers Fired By Trump: ‘You Were the Best Of Us’—Billboard
Ruling on US Affordable Care Act Maintains PrEP Coverage…For Now
Last Friday, the US Supreme Court issued a ruling, which affirmed the constitutionality of the Affordable Care Act’s (ACA aka ‘Obamacare’) preventive services mandate, which means that insurance providers must continue to cover prevention, including for HIV pre-exposure prophylaxis (PrEP), at no cost to patients. The ruling allows preventive care protections to remain in place while the case continues through the courts.
IMPLICATIONS: This ruling helps maintain critical protections that millions in the US rely on for equitable access to HIV prevention and other essential health services. However, the “victory [ruling] must be tempered by what has happened with the CDC’s Advisory Committee on Immunization Practices (ACIP), as it could be a harbinger of what a Secretary of HHS can do to twist committees and task forces that should be composed of technical experts guided by science to ones that are guided by ideology, illogic and political whim,” AVAC said in a statement.
READ:
- Justices Uphold Preventive Care Provision in Affordable Care Act—New York Times
- Supreme Court Upholds ACA Preventive-Care Protections—AVAC statement
- National Coalition of HIV, LGBTQ+ and Health Advocates Applauds Court Decision Upholding Law that Ensures Access to PrEP and other Healthcare Preventive Services—PrEP4All
- Supreme Court rules to save free access to PrEP and other preventive care—The Advocate
US Supreme Court Ruling Limits Federal Courts’ Ability to Block Presidential Actions
The Supreme Court also ruled in favor of the US President in the case, Trump v CASA, significantly limiting the power of federal judges to issue nationwide injunctions that block a law or executive branch action while it’s being challenged in federal court. The case was brought in response to the President’s executive order attempting to deny birthright citizenship to children of undocumented immigrants and temporary visa holders. Federal courts blocked this order as unconstitutional. However, this ruling by the Supreme Court will only allow federal courts to block such policies for the parties directly involved in the lawsuits, not nationwide.
IMPLICATIONS: This ruling is a major victory for the executive branch. It removes a key tool used to halt federal actions that threaten health and rights and puts limits on the checks and balance of executive power. It will make it more difficult for plaintiffs to use federal courts to block harmful policies until broader legal challenges can progress. Dissenting Supreme Court justices warned the decision poses a grave risk to accountability, allowing unconstitutional actions to proceed unless each harmed party sues individually.
READ:
- A blow to judicial power and a win for Trump—The Economist
- Supreme Court hands Trump major win, limits judges’ ability to block birthright citizenship order nationwide—Politico
WHO Announces New Leadership
WHO Director-General Tedros Adhanom Ghebreyesus announced a new leadership team of 36 directors (down from 74), including the appointment of Tereza Kasaeva, former TB lead, to head the newly combined HIV, TB, hepatitis, and STI department. Meg Doherty, who formerly headed the HIV Department, was named as Director of the Department of Science, Research, Evidence and Quality for Health. Significant staff reductions across WHO as well as a significant shift from Geneva to the field are planned in the months ahead. This restructuring is part of a broader effort by WHO to streamline leadership in response to its $1.7 billion shortfall, stemming from the pullout of the US government’s contribution and pressure to decentralize operations.
IMPLICATIONS: The merging of these disease areas under a single department comes at a critical time for both the HIV and TB responses, with prevention and treatment gains at risk due to shifting ideological priorities from the US government. It will be essential for advocates to monitor this transition, ensure technical expertise is retained within the combined department, champion comprehensive and integrated HIV and TB programs within WHO’s agenda, and ensure progress toward epidemic control does not stall.
READ:
- EXCLUSIVE: WHO Chief Names New Team of Directors – Mostly Familiar Faces—Health Policy Watch
New Issue of PxWire
This issue looks at the scale of shuttered prevention programs for key populations (KPs), the potential market for injectable lenacapavir for PrEP, and the devastating cuts to research for an HIV vaccine.
What We’re Reading
- Expanding access to a choice-based HIV prevention market—Journal of the International AIDS Society
- PEP in Africa: prospects, opportunities and challenges—Journal of the International AIDS Society
- Building a resistance to US assaults on public health—The Lancet
- Over 300 NIH employees go public with their concerns about what’s happening to that organization—Federal News Network
- William Haseltine discusses cuts to federal funding for scientific research—NPR
- ‘I don’t want my boy to be positive’: pregnant women face sky-high viral loads as cuts hit HIV care in Africa—The Guardian
- RFK Jr. says medical journals are ‘corrupt.’ As former NEJM editors, we know he’s wrong—STAT
- Seven Chaotic Months in the Life of a New Federal Judge—New York Times
- The Reagan-Appointed Judge Fast-Tracking Trump to Trial—New York Times
Updated Resources
- Principles of a responsible transition of American leadership to end AIDS: Strategic transition or pandemic resurgence?, Friends of the Global Fight Against AIDS, Tuberculosis and Malaria
- Global Fund Replenishment 8 Scenarios, DataEtc.
- Science & Community Impacts Mapping Project (SCIMaP), SCIMaP
- The Scientific Journey of Lenacapavir, AVAC
- LEN Generics — Can we go faster?, AVAC
- Lenacapavir Regulatory Approval, AVAC
- Research Matters, AVAC, HIVMA, TAG
- HIV Prevention R&D at Risk, AVAC