As many global health fields reassess their reliance on US government funding for research and development, the STI field—already underfunded and reliant on alternate donors—now faces even greater uncertainty. In this newsletter, we share a new STI resource for advocates and highlight the top issues we’re monitoring as events continue to unfold.
STIWatch Quarterly Newsletter
At A Glance: The MPT R&D Pipeline
This graphic shows the status of products in development.
Spotlight on MPTs Addressing STIs
This graphic outlines the development journey of multipurpose technologies (MPTs) that guard against STIs, including HIV, while also preventing pregnancy. It tracks the advancement of various potential products through different trial stages, emphasizing their combined protective roles.
Sexually Transmitted Infections: ‘Self-testing’ versus ‘self-collection’: the critical role of consistent language in the field of STI diagnostics
This editorial from AVAC’s Alison Footman and colleagues makes the case for precise and consistent language around self-testing and self-collection. because clarity impacts policy, expectations, and access.
The Real-World Impact of Defunding STI Research
The US presidential administration’s funding cuts and policy shifts are reshaping the public health landscape in profound ways. While many of these changes have drawn significant media attention, the impact on sexually transmitted infection research and prevention has remained largely overlooked, though the consequences are dire, writes AVAC’s Alison Footman writes in TheBodyPro.
Global R&D Funding for HIV and STIs
As STI rates rise and global funding falls, the path forward lies in local leadership, new funding models, inclusive policymaking, and smart integration. In data presented at the STI & HIV World Congress by AVAC and Impact Global Health, a 127% growth in STI R&D funding between 2018 and 2023 from $96m to $218m was promising, but overly dependent on US governments. This growth is likely not sustainable given recent shifts in US policies, and developers, funders, advocates, and other stakeholders must stay accountable to ensure new tools are community-centered and truly address prevention, detection, and treatment needs.
Worldwide Prevention, Shared Protection
This Issue Brief describes the impacts of the elimination and reduction of funding that supports sexually transmitted infection (STI) research, testing, and prevention programming. This funding is critically important as STI rates continue to increase globally with more than 1 million curable STIs, including chlamydia, gonorrhea, syphilis, and trichomoniasis, acquired every day. Without appropriate testing, treatment, and prevention programs, there is a risk that STI rates will continue to increase leading to more cases of infertility, pelvic inflammatory disease, and cancers. Further, there is the risk that we will lose the ability to monitor, react to, and prevent the rise of antimicrobial resistant gonorrhea, which has become an issue for the majority of treatment options currently available.
Self-Care Advocacy for HIV and STI Prevention
Self-care, the ability of people to promote and maintain health, prevent disease, and cope with illness and disability with or without the support of a healthcare worker is especially critical now, as the new US administration’s sweeping funding cuts and policy shifts threaten to erode support for traditional healthcare services, including HIV and STI programs.
By putting testing, prevention, and treatment directly into people’s hands, self-care can help communities maintain vital health services despite reduced funding, limited access to healthcare, and diminished government support. Read our new guide, Self-Care Advocacy for HIV and STI Prevention, on STIwatch.org.
STIWatch Newsletter, November 2024
The past few months have brought exciting developments in the field of Sexually Transmitted Infections (STIs). The World Health Organization released the global priorities for STIs, which included the need to develop low-cost, rapid, STI point-of-care tests, vaccines, and communication strategies to increase STI awareness, prevention, service engagement. But we continue to see a soaring rise in STI incidence and an underfunded infrastructure for researching new treatment, prevention and testing tools. It’s up to the global community to ensure that research and development continues to see funding for better, faster, less expensive tools to reduce the toll of STIs. Read on for resources and insights to guide your advocacy.
Conference updates
The annual conference of the International AIDS Society in July highlighted the troubling trend in the soaring rise in STI incidence. A preconference, Mobilize for Action on Sexually Transmitted Infections addressed the urgent need to confront the global spike in STI rates, particularly syphilis, gonorrhea, chlamydia, and trichomoniasis. Dr. Jeanne Marrazzo, director of NIAID, when discussing the number of global deaths from syphilis, which is treatable, said, “I think some of the more staggering statistics here, in addition to the sheer number of new infections, is the fact that we had in 2022 over 200,000 syphilis-associated deaths, which to me is practically medieval.” The meeting hosted a rigorous debate exploring the question of implementing DoxyPEP, given there’s no efficacy data for cisgender women in light of fears of creating drug resistant strains of STIs from wider use of doxycycline. Strong arguments were made on both sides, but this controversy is one of the reasons AVAC recently published an Advocate’s Guide to Doxycycline to Prevent STIs.
The STI Prevention Conference in Atlanta, Georgia convened attendees in-person for the first time in four years and discussed the rise in syphilis rates, emerging gonorrhea treatments, STI funding and policy initiatives, and doxycycline post-exposure prophylaxis (DoxyPEP). Notably, much discussion centered on NIAID’s support for a biorepository to advance diagnostic development. This biorepository would streamline access to necessary specimens to support the research and development of new diagnostics.
Save the date

New resources
Discover STIWatch.org!
STIWatch.org is an updated platform designed to enhance understanding and advocacy for STI vaccine and diagnostics research, development, and rollout. It offers comprehensive information on common STIs, a clinical trials dashboard, advocacy priorities, and a range of resources and tools to support STI prevention and treatment efforts.
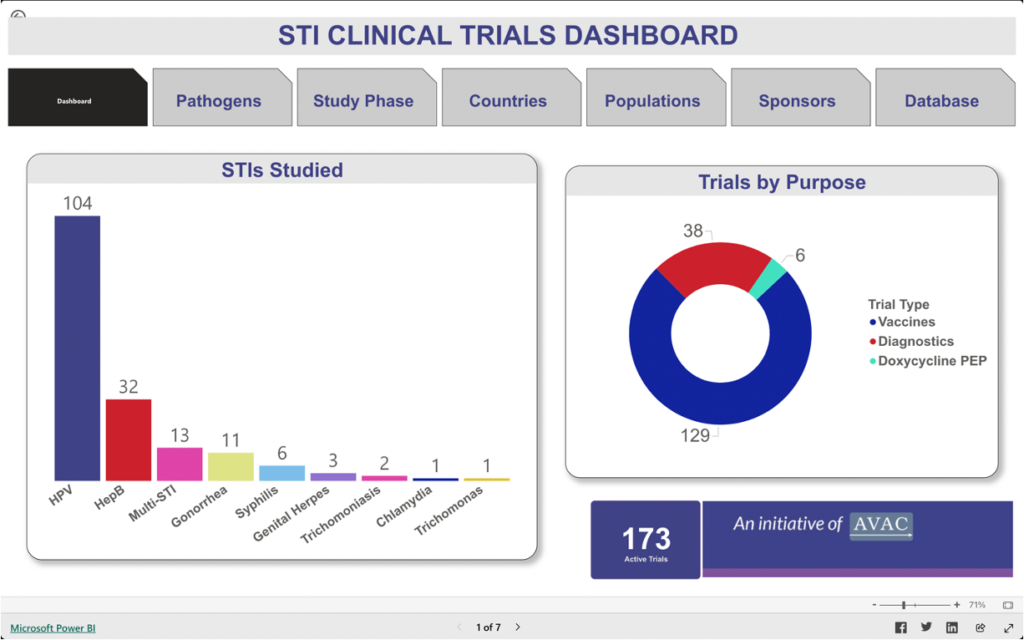
STI Clinical Trials Dashboard
This dashboard provides information about trials focused on vaccines, diagnostics, and the use of doxycycline post-exposure prophylaxis (DoxyPEP) to detect, treat, and prevent chlamydia, gonorrhea, hepatitis B, herpes simplex virus (HSV), human papillomavirus, syphilis, trichomoniasis infections, and Mycoplasma genitalium.
Webinar Summary
Regulatory Pathways to Promote Access to STI Diagnostics. This webinar co-hosted with World Health Organization (WHO) supported researchers, product developers and the global advocacy community in identifying and discussing ways to bring new STI diagnostics to market with speed, equity and scale.
What We’re Reading
- Special Issue: Sexually Transmitted Diseases. This leading journal celebrated 50 years by publishing a series of editorials that review the careers of some of the leading experts in the field. These stories are both inspiring and enlightening, highlighting the decades of efforts and growth in addressing STIs. AVAC’s own, Alison Footman, PhD, wrote about her experiences in the STI field and how instrumental mentorship has been in her career growth.
- Gonorrhea point-of-care diagnostics technology and market landscape. This landscape report provides an overview of gonorrhea point-of-care diagnostics that can be offered closer to patients and communities and limit time to results and ultimately treatment. Gonorrhea touches on multiple public health priorities including the need to reduce STI rates, growing antimicrobial resistance, and its impact on sexual and reproductive outcomes and HIV transmission.
- FDA Marketing Authorization Enables Increased Access to First Step of Syphilis Diagnosis. The US Food and Drug Administration approved the first syphilis self-test. This is a monumental step in expanding syphilis testing options as infections have increased drastically over the past five years. Self-tests can provide people with the option to learn their syphilis status and seek additional testing and treatment options from a healthcare provider.
Partner Spotlight

AVAC partners have been busy moving the needle to improve STI prevention, testing, and treatment options in their respective countries. The Latu Human Rights Foundation partnered with HEP Initiative Zambia on a symposium to foster ideas on how to better address viral hepatitis and integrate hepatitis B interventions into other government funded health programs.
To learn more about AVAC’s STI Program, visit STIWatch.org and avac.org/sti. Email [email protected] for questions or additional information. And to sign up for specific updates on STIs, click here.
Advocates’ Guide to Multipurpose Prevention Technologies (MPTs)
Multipurpose prevention technologies (MPTs) are products designed to simultaneously address more than one sexual and reproductive health concern. This advocates’ guide shows the pipeline of products in development, discusses why MPTs are needed, investment, and what advocates can do to push for MPT development and introduction.