Contact: [email protected]
NEW YORK, NY, June 5, 2025 — AVAC denounces recent proposals and actions by the US administration that signal a clear intention to defund and eliminate lifesaving global health research, development and delivery programs. If passed by Congress, proposed funding rescissions for the current year’s budget would claw back billions of Congressionally appropriated dollars for critical, life-saving programs. In addition, the President’s Fiscal Year 2026 (FY26) budget proposal would further cut funding and entrench a wide range of anti-science and anti-public health policies, many of which undermine the rights of communities vulnerable to HIV. AVAC urgently calls on Congress to step-up in bi-partisan support that aligns the US federal budget with evidence and delivers impact.
“These actions are doing irreparable harm to health research and programs that form the backbone of global efforts to end HIV,” said Mitchell Warren, AVAC’s executive director. “This is not just a budget proposal; this is a shortsighted and reckless policy roadmap that provides further proof that this administration has no regard for science, research, or public health. Every day of unchecked executive overreach unravels decades of progress. Congress must fulfill its duties and intervene to protect policies and programs that have made Americans and the world safer, healthier and more prosperous.”
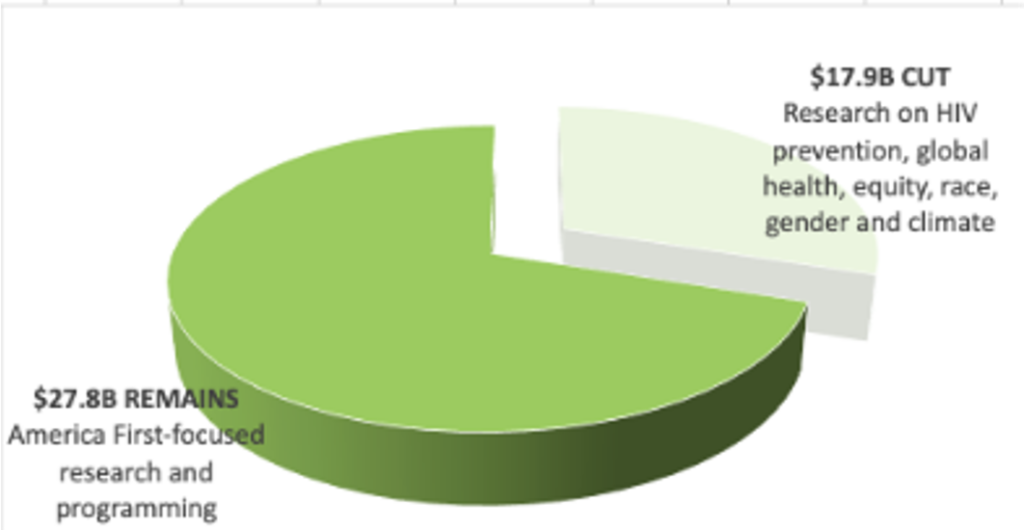
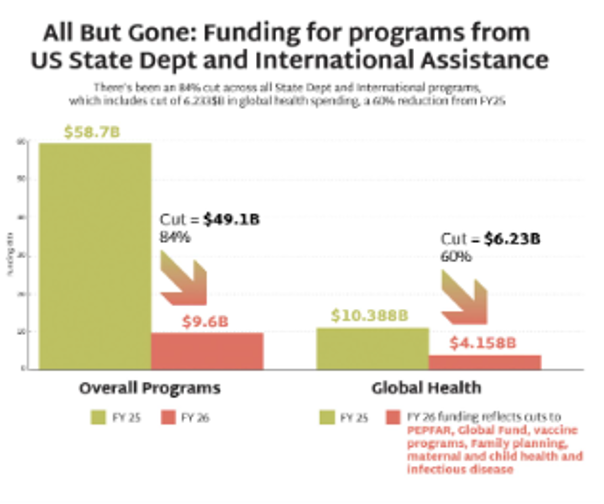
Released in May as a “skinny” version, the President’s full FY26 budget proposal would dismantle the architecture for global health, including programs and research with historically broad bipartisan Congressional and public support. The FY26 budget proposes slashing PEPFAR by 34% and National Institute of Allergy and Infectious Diseases (NIAID) funding by 36%, for a combined total of over $5.5 billion in cuts – potentially crippling HIV programs and research. The FY26 budget further targets the Global Division of HIV and Tuberculosis (DGHT) at the US Centers for Disease Control and Prevention (CDC) by eliminating it along with other global health programming at the agency. PEPFAR is severely weakened without the partnership of CDC’s global health division and USAID, which bring vast expertise and technical assistance in the implementation of programs at the country-level.
Additionally, the proposed rescissions package would eliminate over $900 million from FY25 global health programs. The rescissions package would cancel not-yet-spent funds, and the administration has not excluded PEPFAR from these further reductions. If passed by Congress, it would codify, or make legal, the unlawful dismantling of USAID, which was initiated through presidential executive overreach and reckless actions by DOGE across federal agencies. Just as important, harmful, ideological rhetoric across FY26 budget documents and the rescissions package attempt to justify targeted cuts to services for the LGBTQI+ community, family planning and reproductive health. Such policies are antithetical to a rights-based public health approach to meeting critical needs among communities who are the most marginalized and vulnerable to HIV and other diseases.
These actions by the administration also come on the heels of last week’s announcement of the elimination of NIH funding for the Consortia on HIV/AIDS Vaccine Development (CHAVD). Founded in 2005, the CHAVD programs – based at Duke University and Scripps Research Institute – have been instrumental to advancing HIV vaccine research and development, contributing to progress toward an HIV vaccine and other scientific innovations.
“A shuttered CHAVD imperils the ongoing quest for an HIV vaccine and sidelines scientific discovery at large, leaving some of the most accomplished scientists in the world without the federal resources needed to continue vital research,” said Stacey Hannah, director of Research Engagement at AVAC. “Americans need to be reminded that vaccines are one of, if not the most cost-effective, impactful health interventions. The CHAVD cuts represent an attack on fundamental science that protects the well-being of all and boosts prosperity in our country and the world.”
These actions are part of a broader anti-science agenda from this administration, which has already taken steps to severely constrain the work of the HIV Prevention Trials Network, the HIV Vaccine Trials Network, the Adolescent Medicine Trials Network for HIV/AIDS Intervention, and the Advancing Clinical Therapeutics Globally for HIV/AIDS and Other Infections (ACTG).
“In a cruel irony, these combined cuts come just as the field reaches a moment of historic promise in HIV prevention,” said John Meade, senior program manager for Policy Advocacy at AVAC. “Later this month, the US FDA is expected to approve lenacapavir (LEN) as a twice-yearly injectable form of PrEP. This product represents the culmination of decades of investment in all stages of scientific innovation, including basic science and global research infrastructure, especially in South Africa. Without NIH investments over the past two decades, the world would not be on the cusp of approval and introduction of LEN for PrEP.”
AVAC calls on Congress to exercise its power of the purse under the Constitution by rejecting the President’s proposed cuts to global health, research, development and science. Congress must maintain this critical funding, which makes the world safer, healthier, and more prosperous. Congress should also immediately reject the rescissions package in its vote expected next week and do all it can to restore funding for HIV research and programming across the federal government.
The field must urgently make the case for sustained investment. Everyone concerned about the devastating effects of ongoing and threatened cuts should reach out to their Senators and Congresspeople immediately. Call the Capitol switchboard at (202) 224-3121, or reach out to your Senators online and Representatives directly.
###
About AVAC
AVAC is an international non-profit organization that provides an independent voice and leverages global partnerships to accelerate ethical development and equitable delivery of effective HIV prevention options, as part of a comprehensive and integrated pathway to global health equity. Follow AVAC on Bluesky and Instagram. Find more at www.avac.org and www.prepwatch.org.