This graphic outlines the development journey of multipurpose technologies (MPTs) that guard against STIs, including HIV, while also preventing pregnancy. It tracks the advancement of various potential products through different trial stages, emphasizing their combined protective roles. Excerpted from our Advocates’ Guide to Multipurpose Technologies.
Spotlight on MPTs Addressing STIs
AVAC’s Guide to HIVR4P 2024 in Lima
We are looking ahead to the biennial HIV Research for Prevention 2024 conference in Lima, Peru next week, 6-10 October. HIVR4P is a space where biomedical HIV prevention research, policy and programs takes center stage. Whether you’ll be in Lima or are following from afar, AVAC will keep you connected!
Read on for information on AVAC sessions, a sortable roadmap, the Advocates’ Corner (open all week) and more!

Resources
- Use AVAC’s Prevention Roadmap of conference sessions and satellites to find what interests you the most. You can download it as a sortable spreadsheet or PDF.
- Advocates’ Corner: If you plan to be in Lima, be sure to join us and our CASPR partners at the Advocates’ Corner to take the conversations and themes deeper. The Advocates’ Corner will be open throughout the conference hosting a program of activities along with materials displays and opportunities for informal networking. Be sure to check the events page for updates on programming.
- AVAC’s Coverage: From the latest news on injectable lenacapavir, to updates on the development of next generation prevention options, to the complex work of implementing the tools that exist today and all the advocacy needed to get it all done, our email dispatches to the Advocates’ Network keep you informed. Follow events in real time on Twitter at #HIVR4P2024 and Instagram.
- People’s Research Agenda: During HIVR4P, we’ll be releasing the new People’s Research Agenda, a global initiative driven by communities and advocates to define the most urgent priorities, research questions and recommendations for HIV prevention research. We hope it serves as a guide to what is – and should be – discussed at HIVR4P and beyond.

Satellites and Sessions Featuring AVAC and Partners
Sunday, 6 October
- Satellite: Creating a Menu of Options: Early R&D of HIV prevention products for women, the MATRIX way, 08:30 – 10:00
- Satellite: The Continued Relevance of HIV Vaccines in the Age of Long-Acting Antiretrovirals: Insights from India and Sub-Saharan Africa, 10:30 – 12:00
- Satellite: Enhancing service providers’ engagement in PrEP delivery, uptake, and retention in Latin America & the Caribbean, 10:30 – 11:30
- Satellite: The brightest under 30: Celebrating youth voices and promoting meaningful youth engagement in HIV prevention research, 10:30 – 12:00
- Satellite: Catalyzing Progress in the Inclusion of Pregnant and Lactating People in HIV Prevention Research, 12:30 – 14:00
- Satellite: Delivering on the promise: Defining optimal implementation strategies and service delivery packages for the Dual Prevention Pill,14:30 – 16:00
- Satellite: What’s Really Going to Work in the Lives of AGYW? Innovations in Acceptability and Mobile Health Support Interventions for HIV Prevention, 12:30 – 14:00
- Satellite: Understanding the role and power of advocates and researchers in advancing Discovery Medicine Vaccine Trials (DMVTs) and the development of Broadly Neutralizing Antibodies (bnAbs) for HIV Prevention, 16:30 – 17:30
Monday, 7 October
- Satellite: Leveraging SBR to Engage and Empower communities in HIV Prevention Research, 09:00 – 10:00
- Satellite: Manifest Choice: Enabling a future free of HIV, 10:30 – 11:30
- Satellite: Advancing Good Participatory Practices (GPP) in Research: Enhancing Community Engagement for Impact, 12:30 – 13:30
Tuesday, 8 October
- Oral abstract: The big picture: Global trends in HIV prevention, 11:00 – 12:30
AVAC’s Catherine Verde Hashim will present the abstract, Identifying global typologies of HIV PrEP implementation: an analysis of global data using PrEP-to-need ratios and PrEP distribution volumes. - Oral abstract: Novel antiretrovirals and formulations for prevention, 11:00 – 12:40
Jim Pickett will co-moderate this session which will discuss new data on islatravir and lenacapavir for PrEP, U=U and more.
- Symposium: Quo vadis: Future design and conduct of vaccine and bNAb clinical trials, 13:30 – 15:00
AVAC’s Grace Kumwenda and colleagues will discuss the viability and practicality of bNAbs as HIV prevention tools.
- Symposium: Prevention product profiles for future options, including long-acting PrEP formulations and products, 13:30 – 15:00
Moderated by Mitchell Warren, this session will discuss the issues of choice and combination products in HIV prevention, and will look at Target Product Profiles for various technologies, especially long-acting PrEP options.
Wednesday, 9 October
- Symposium: Reducing burdens and barriers to expand the use of HIV prevention options, 13:30 – 15:00
This session will explore the promise, potential and risks of using remote tools, such as telemedicine, virtual tools, apps and self-testing and the impact of other tools used to expand access and uptake of HIV prevention modalities. It will also review approaches to overcome misinformation and mistrust.
Thursday, 10 October
- Oral abstract: Policy and legal barriers to HIV services, 08:30 – 10:00
Brian Minalga of Fred Hutchinson Cancer Center will present, The transgender scorecard: ensuring representation in HIV prevention research.
- Oral abstract: Driving PrEP implementation through community engaged science, 13:00 – 14:30
Esther Nakkazi will present, Using local languages for accurate science reporting in Media Science Cafés in East and Southern Africa.
Find these resources, conference highlights and more at AVAC’s dedicated HIVR4P 2024 page. And watch this space for new opportunities to come together and shape what happens next.
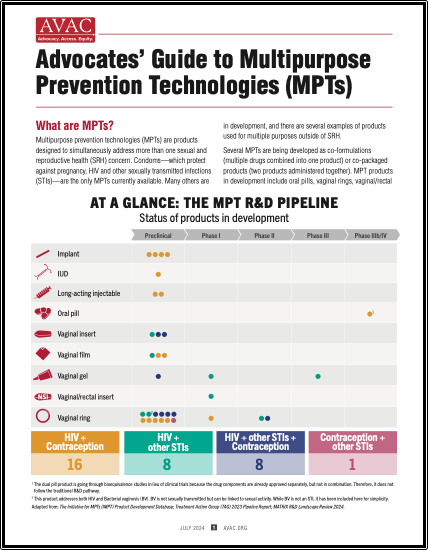
At A Glance: The MPT R&D Pipeline
This graphic shows the status of products in development. Excerpted from our Advocates’ Guide to Multipurpose Technologies.
Advocates’ Guide to Multipurpose Prevention Technologies (MPTs)
Multipurpose prevention technologies (MPTs) are products designed to simultaneously address more than one sexual and reproductive health concern. This advocates’ guide shows the pipeline of products in development, discusses why MPTs are needed, investment, and what advocates can do to push for MPT development and introduction.
SRH + HIV integration advocacy, Pandemic Accord, GPP and more!
AVAC’s round-up of resources, updates and insights this week includes a new roadmap for sexual and reproductive health (SRH) and HIV integration, resources to support an equitable Pandemic Accord, innovations in Good Participatory Practices (GPP) and more!
The power of choice in contraception, sexual health and HIV prevention this World Contraception Day
Roadmap: Sexual and Reproductive Health (SRH) Integration Roadmap

Copper Rose Zambia, as a part of CASPR and AVAC launched a new resource addressing the critical need for integrated SRH and HIV services. This roadmap provides key steps for success, focusing on collaboration, strategic mapping and targeted advocacy.
Advocate’s Guide: Advocates’ Guide to Multipurpose Prevention Technologies (MPTs)
MPTs are products designed to simultaneously address more than one sexual and reproductive health concern. This advocates’ guide shows the pipeline of products in development, discusses why MPTs are needed, investment, and what advocates can do to push for MPT development and introduction.
What will it take for an equitable Pandemic Accord?
Call to Action: Pandemic Accord Priorities from the Coalition of Advocates for Global Health and Pandemic Preparedness

A group of organizations advocating for an integrated and holistic approach to preparedness that emphasizes equity, inclusion, and synergies of multiple global health programs in advancing preparedness, shares five priorities in Pandemic Accord negotiations.
UNGA Side Event: Restrategizing Civil Society Engagement for Pandemic and Global Governance

AVAC’s Sam Rick moderated CISDI’s event alongside Nina Schwalbe, Lawrence Gostin, Eloise Todd and others, reminding the audience that for pandemic prevention, preparedness and response (PPPR) to succeed, lessons from the HIV response must be integrated into the architecture being built for PPPR.
Good Participatory Practices in action
Call for Applications: Now Accepting Applications for the 2024 Good Participatory Practice Online Course
The 2024 Good Participatory Practice Online Course is now accepting applications for 30 spots! This course offering will run 14 October – 20 December 2024. The application deadline is 9 October.
Recording: Innovations in GPP

Recording / Clever Chilende Slides / Sarah Read Slides / Ntando Yola Slides
Harnessing private sector strategies for family planning to deliver the Dual Prevention Pill, the first multipurpose prevention technology with pre-exposure prophylaxis, in an expanding HIV prevention landscape
Working with colleagues, AVACers Cat Verde Hashim and Kate Segal published this research article in the Journal of the International AIDS Society. The researchers undertook qualitative research in Kenya, South Africa and Zimbabwe to prioritize private sector service delivery approaches for the introduction of the DPP.
First Full Day of AIDS 2024
Lenacapavir for PrEP has taken center stage at the 25th International AIDS Conference, #AIDS2024, which opened Monday with many highlighting its potential for long-acting PrEP for HIV prevention. Some advocates took to the halls in protest calling on LEN’s maker to price the product low. Leaders across HIV voiced the need for urgency in galvanizing support for the introduction of lenacapavir. “It is gobsmackingly exciting to see zero in a clinical trial” AVAC’s Mitchell Warren told Forbes. The potential to bend the curve of the epidemic depends on speeding access to prevention options like LEN, that show high efficacy.
At the same time, it’s imperative to remember that neither lenacapavir, nor any other single product, now or in the future, will ever be a ‘miracle drug’, and LEN must not be equated with a vaccine, as seen in some conference media reports.
Ongoing investment in the pipeline for HIV prevention must be founded on the principle of choice, offering a range of products to meet diverse needs among people facing the risk of HIV. We hope that vaccines will one day be among those choices, as will long and short-acting products, and topical and systemic products. Clear communication that allows product users to understand how products are different supports widespread adoption of HIV prevention and moves the world toward finally ending the epidemic.
See AVAC’s statement calling for early planning to accelerate LEN’s regulatory review and for ambitious introduction plans, and the joint civil society call to action with specific priorities about what needs to happen next. Our primer, the Lens on LEN, also offers advocates a guide in explaining the findings from the Purpose 1 trial and next steps for advocacy.
As Albert Liu from UCSF’s Center for AIDS Research told delegates in the symposium on breakthrough and insights in long-acting technologies, “It’s never just the ‘product.’ New options can’t solve everything.” Atul Gawande of USAID reiterated a similar message at the satellite focused on women’s prevention, “The critical message to understand is that there isn’t going to be a magic bullet for prevention. What we have to understand is that there are also considerations that affect the likelihood that women will have what they want and what they’re likely to use.”

People First
The conference theme, “Put People First,” is the main message we all must hear. Lillian Mworeko of the International Community of Women living with HIV/AIDS East Africa (ICWEA) captured the essence of the meeting: “I am not just a recipient of care. I need a seat at the table to meaningfully engage and tell people what I need and how I need it.”
Monday’s opening session underscored the vital role of community engagement and the necessity for inclusive policies that address disparities affecting marginalized populations. Jay Mulucha of Fem Alliance, Uganda, the first trans man to speak at the International AIDS Conference, delivered a compelling message to the 12,000+ delegates attending both in person and online: “As a trans man living in Uganda, I am asking you to stop leaving us behind. Nothing about us, without us.”

New UNAIDS Report
UNAIDS released a report, The Urgency of Now, AIDS at a Crossroads calling out funding disparities and the need to dismantle the discrimination and stigma that are pushing the most marginalized people away from health care. The report warned of the peril in delayed funding decisions; investment needs to happen urgently for long-acting treatment and prevention options to reach all low- and middle-income countries and meet 2025 targets.
Money, Money, Money
Making the most of investment in HIV prevention fundamentally depends on political will, but the field needs the right data, too. Monday’s satellite session, Money, money, money: Building towards a sustainable end state for HIV prevention, called for better data that goes beyond PrEP initiation numbers. “[PrEP initiations] alone do not tell us how much product is needed or how long people stay on PrEP. We are not collecting the right data,” said Katherine Kripke of Avenir Health. AVAC’s Mitchell Warren described the vicious cycle of small pilot projects generating limited data on PrEP use, resulting in unpredictable demand and cautious investment. “We have lots of small examples, and then we don’t scale it up because governments don’t know what it will cost. And still the world has 1.3 million new infections. We have to break the cycle.”
The Future of Women’s Prevention
At the session organized by CASPR (Coalition to Accelerate & Support Prevention Research) and MATRIX (Microbicide R&D to Advance HIV Prevention Technologies through Responsive Innovation and Excellence), New ways for the next wave: Innovative R&D for the future of women’s prevention, MATRIX laid out their innovative approach that involves very early engagement of all stakeholders in the research, development and delivery of new products for HIV prevention. The session emphasized the equitable inclusion of women in all phases and in every aspect of R&D—as researchers, potential users, and advocates.

Sharon Hillier of Magee-Women’s Research Institute noted, “What we’ve learned in our research is that women care about efficacy, but it’s just one element of what they consider when they decide on prevention. They’re quite interested in safety, ease of use, discretion, price, availability, and accessibility.”
Stay tuned for more highlights from AIDS 2024 and visit our curated conference webpage, which includes new resources and summaries of the preconference sessions.
MPT R&D Funding 2018-2021
This graphic tracks funding levels for a variety of multipurpose technologies for several years. Excerpted from our Advocates’ Guide to Multipurpose Prevention Technologies.
PxPulse: The Advocacy Chronicles with Ruth Akulu

On this episode of the Advocacy Chronicles: a look at advocacy in Uganda for the Dual Prevention Pill (DPP), a new product combining oral PrEP and oral contraception.
Ruth Akulu is a Youth Representative of the Uganda Country Coordinating Mechanism Board for Global Fund, member of the DPP Civil Society Advisory Group, and a 2022 AVAC Advocacy Fellow. Akulu talks about her work to mobilize regulatory authorities to prepare for the DPP. And while she was at it, the establishment of a groundbreaking new initiative, the Product Regulator’s Engagement Committee, which is supporting ongoing engagement between government regulators and young women representing their communities.
Listen to learn why the DPP is a priority for young women and HIV prevention, the challenges Ruth confronted and tactics that supported the success of this advocacy.
Listen
Resources
- The Advocates’ Guide to Multipurpose Prevention Technologies
- The Dual Prevention Pill page on PrEPWatch.org
- FAQ on the Dual Prevention Pill
- Market Preparation and Introduction Strategy for the DPP (Executive Summary also available)
- Frontiers in Reproductive Health special issue: Multipurpose Prevention Technologies
Avac Event
AIDS 2024: New Ways for the Next Wave: Innovative R&D for the future of women’s prevention
This session will be 9:30AM to 11:00AM Munich time.
Women need a range of HIV prevention options to meet different needs, preferences and life circumstances. Currently available pills, rings and injectables are necessary but insufficient. How might we accelerate the delivery of methods we have while developing additional ones – including systemic and non-systemic methods, short-acting and on-demand products, to complement longer-acting ones? Product developers and advocates are collaborating on new approaches to expedite R&D of additional HIV prevention options. Notably, MATRIX is taking a unique approach, endorsed by the CASPR network of advocates, that aims to improve the odds of success of new products – through development, delivery and use.
This session will focus on new efforts to identify and develop promising options, strategically engage all stakeholders, decolonize R&D and involve potential users in all their diversities throughout the process.
Speakers:
- Jeanne Marrazzo, National Institute of Allergy and Infectious Diseases
- Sharon Hillier, University of Pittsburgh / Magee-Womens Research Institute
- Thesla Palanee-Phillips, Wits RHI
Followed by a moderated panel with:
- Chimwemwe Chamdimba, African Medicines Regulatory Harmonization (AMRH) initiative- AUDA/NEPAD
- Kelly Chibale, Holistic Drug Discovery and Development Centre -H3D, University of Cape Town
- Nyaradzo Mgodi, University of Zimbabwe- Harare Health Research Centre
- Jerop Ruth Limo, Ambassador for Youth and Adolescents Reproductive Health Program (AYARHEP)
Moderators:
- Navita Jain, AVAC
- Kenneth Ngure, Jomo Kenyatta University of Agriculture and Technology
Session Chairs:
- Sharon Hillier, University of Pittsburgh / Magee-Womens Research Institute
- Imelda Mahaka, Pangaea Zimbabwe
This satellite session will be co-hosted by CASPR and MATRIX, with support from USAID and PEPFAR.