With the recent FDA approval of injectable lenacapavir (LEN) for PrEP and the drastic withdrawal of US investment in HIV prevention, the field must reimagine and recommit to getting PrEP rollout right this time AND to sustaining the HIV research pipeline. Research on HIV has brought numerous advances to global health, but controlling, and ultimately ending, the epidemic depends on continued investment in innovation.
This issue of PxWire looks at the scale of shuttered prevention programs for key populations (KPs), the potential market for injectable LEN, and the devastating cuts to research for an HIV vaccine.
Read below or download the PDF version of this issue.
Progress in PrEP Uptake
The PEPFAR stop work orders issued by the US government in January 2025 have devastated the HIV response worldwide, including funding for primary prevention. Sustaining the HIV response and bending the curve of incidence depends on identifying new sources of funding to maintain HIV prevention programs for KPs.
- Guidance issued in February 2025 indicated that PrEP services funded by the US government are permitted only for pregnant and lactating people—meaning that KPs, such as LGBTQ+ individuals, sex workers, and people who use drugs, have lost access to PrEP, unless they are currently pregnant or lactating.
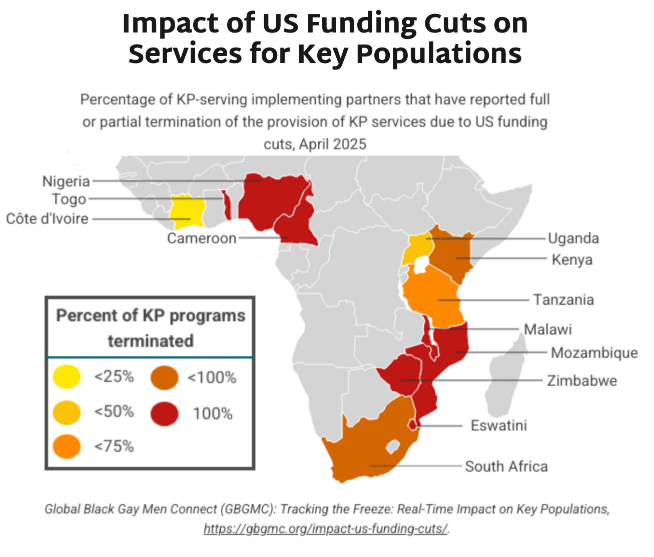
- The graphic below shows key findings on the percentage of KP programs terminated by country. Most of the priority African countries for the HIV response report national KP programs are fully or partially terminated.
- HIV incidence rates amongst KPs are higher than in other populations. Excluding this group from PrEP programming endangers whole communities threatened by HIV, disables the HIV response, and jeopardizes gains made against the epidemic.
- Research undertaken by Global Black Gay Men Connect (GBGMC) examines the impact of US government funding cuts on KP programs in Africa.
- Additional research done by AVAC, in the report Impact of PEPFAR Stop Work Orders, shows that KPs are the group most impacted by US government funding reductions to HIV prevention services worldwide. In some cases, such as Panama, national governments are stepping in to fill the gap.
PrEParing for New Products
Stakeholders—including Global Fund, PEPFAR, WHO, UNAIDS, Unitaid, Ministries of Health, advocates and implementing partners—have critical work to do now to ensure doses of LEN hit the ground as quickly as possible. Check out Gears of Lenacapavir for PrEP Rollout and Getting PrEP Rollout Right This Time to get the details.
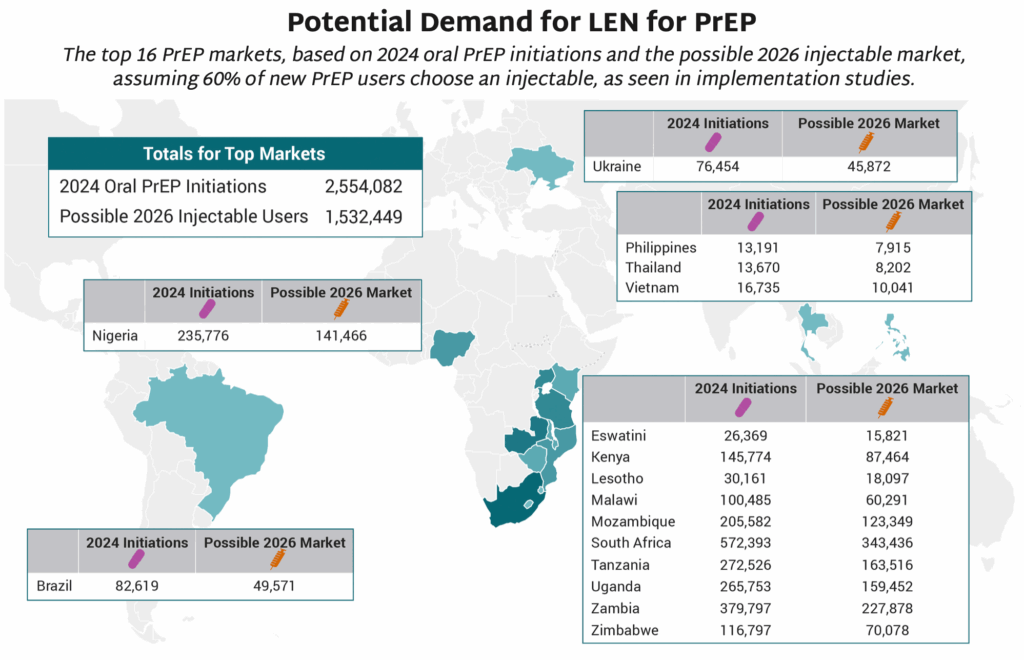
- The map shows 16 countries in Africa, Asia and Latin America that have the largest PrEP markets.
- If total PrEP initiations continue to increase by 20% every year, which is the trend in recent years, and injectables represent 60% of initiations, as seen in implementation studies, 2026 numbers of injectable initiations could be as shown in the map.
- The exact price and volumes of LEN per country is not yet known.
- Of those injectable initiations, LEN is expected to be the majority, given the proposed manufacturing projections from Gilead and the stated ambition of the Global Fund to reach two million people with LEN within the first three years of introduction. Additional volumes of injectable cabotegravir would make up the rest.
- PEPFAR, which in December committed to collaborate with Global Fund, has not yet publicly stated how LEN will fit into their more limited approach to PrEP, which has been restricted to pregnant and breastfeeding women.
The Latest R&D in the Prevention Pipeline
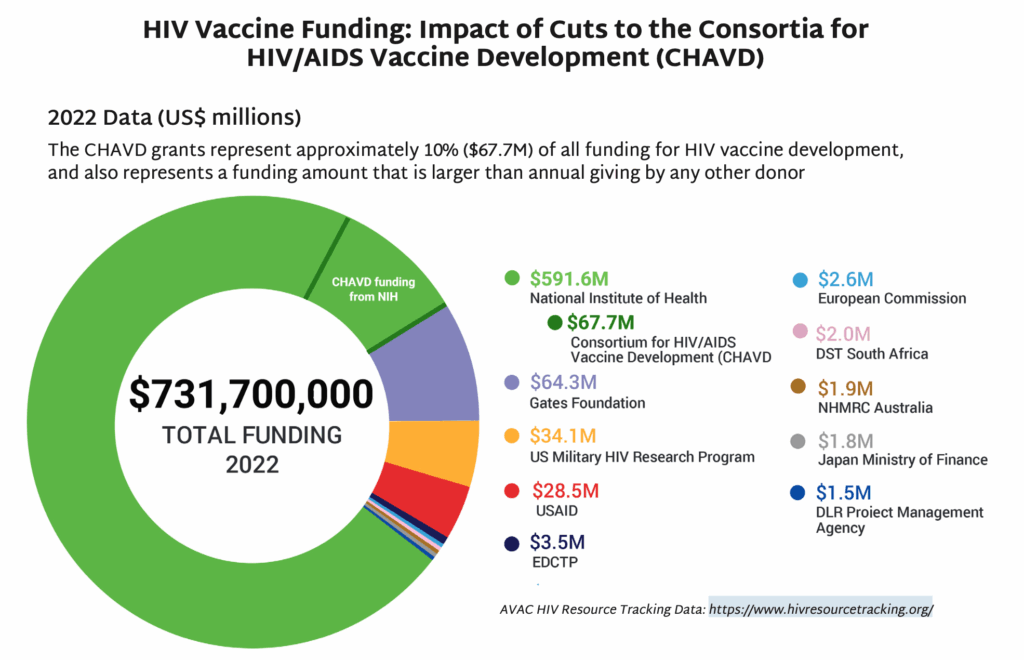
- In May, NIH’s National Institute of Allergy and Infectious Diseases (NIAID) announced that funding for the Consortia for HIV/AIDS Vaccine Development (CHAVD) would end after the current grant cycle in June 2026. With only one more year of funding before the grants end, current plans for research, clinical trials and progress toward a vaccine are all at risk.
- First launched in 2005, the CHAVDs led ground-breaking research to develop an HIV vaccine.
- CHAVD grants currently fund two institutions as consortia leaders—The Scripps Research Institute and Duke University.
- The quest for an HIV vaccine is gaining momentum with field-changing contributions from the CHAVDs. The institutions are currently researching vaccine designs that rely on the immune system’s broadly neutralizing antibodies (bNAbs) to protect against HIV.
- The annual funding for the consortia—approximately $67M—represents a significant chunk of the NIH’s funding for HIV vaccine development, and also approximately 10% of all funding for HIV vaccine research globally each year.
- NIH’s yearly grant total for the CHAVD is larger than any other individual donor’s annual giving for HIV vaccine research. The next closest donor is The Gates Foundation, which donates approximately $64M a year to this research.
- In 20 years of research, CHAVD discoveries have resulted in new technology to combat HIV, influenza, Zika, COVID, and other novel coronaviruses. The loss of the CHAVD will have a devastating impact on the HIV response and scientific discovery.
Prevention Playlist
AVAC develops a wide range of resources to inform decision making and action. Check out the latest:
Join
- CHANGE: In response to the unfolding crisis, more than 1,500 people from civil society organizations around the world have launched CHANGE—Community Health & HIV Advocate Navigating Global Emergencies—a coalition formed to support urgent action: [email protected]
- Subscribe to Global Health Watch: AVAC’s weekly newsletter dedicated to breaking down critical developments in US policies and their impact on global health, at avac.org/global-health-watch
- Fight For Our Lives” Emergency Townhall: Impact of the Trump Administration Foreign Aid Freeze on KP & LGBTQ Communities, Ongoing convening, Register here
Use
- Advocacy Resources for Injectable LEN, AVAC
- HIV Prevention R&D at Risk, AVAC
- Impact of PEPFAR Stop Work Orders, AVAC
- Research Matters Advocacy Toolkit, AVAC, TAG, HIVMA
- Advocates’ Guide: Understanding the President’s Proposed Fiscal Year 2026 (FY26) Budget and Its Implications for Science, Research and Global Health, AVAC
Watch/Listen
- FDA Approves Injectable LEN for PrEP
- Fight for Firewalls: HIV and Health Data Privacy in the Snowballing Surveillance State, Webinar
- Embracing Task Shifting and Innovation to Support Expanded Access to Long-Acting Injectable PrEP, Webinar
- The Scientific Journey of Lenacapavir: From basic science to clinical development to impact, Webinar
- Critical Advocacy: How Civil Society is defending the HIV Response and Global Health, Podcast
- HIV Prevention at a Crossroads: Why we still need an HIV vaccine, Webinar
- A New Era of HIV Prevention: Accelerating access to long-acting prevention options through sustainable prevention systems and financing, Webinar
Read
- FDA Approves Injectable Lenacapavir for PrEP, AVAC
- Trump Aid Cuts Deal a Blow to HIV Prevention in Africa, Reuters
- Will long-lasting HIV preventive be a game changer—or a missed opportunity?, Science
- Regulators Approve a Twice-Yearly Shot to Prevent HIV Infection, New York Times
- FDA Approves Twice-Yearly Lenacapavir for HIV Prevention, POZ
- FDA approves twice-yearly shot for HIV prevention, Helio
- BREAKING: FDA approves breakthrough drug that reduces risk of contracting HIV by 96 percent, The Advocate
- Getting PrEP Rollout Right This Time: Lessons from the field, AVAC
- Why STI Funding Matters, AVAC
- AVAC Condemns Removal of the Advisory Committee on Immunization Practices, AVAC
- AVAC Denounces White House Effort to Codify DOGE Cuts to Health, Research and Foreign Assistance, AVAC
- Protect Federal Funding for HIV, TB, and STI Research and Prevention at the National Institutes of Health, AVAC
- The People’s Research Agenda, AVAC
- Worldwide Prevention, Shared Protection: Why STI Funding Matters, AVAC

