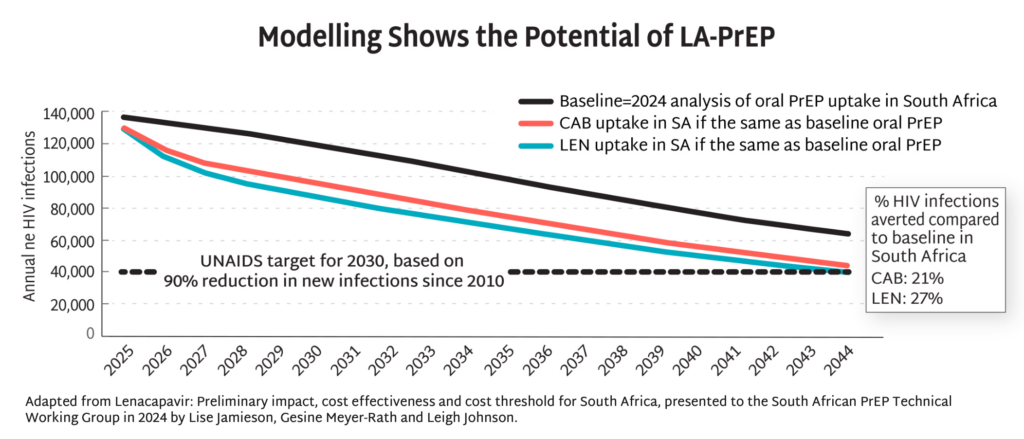
Modelling data from South Africa demonstrate the potential of injectable PrEP to dramatically reduce HIV incidence by up to 90% by 2044, and potentially even sooner with more aggressive uptake. This potential goes beyond South Africa, lighting the way toward epidemic control the world over. Excerpted from PxWire.
Modelling Shows the Potential of LA-PrEP
PrEP Delivery Imperiled
Programs for delivering PrEP have been shuttered all over the world by the withdrawal of the US government from global health. This graphic illustrates some of the severe measurable impacts of these cuts. Excerpted from PxWire.
PxWire Volume 15, Issue 2
The field of HIV prevention is confronted with two opposing forces; programs for delivering PrEP have been shuttered all over the world by the withdrawal of the US government from global health. At this same moment in history, next-generation long-acting products hold great promise to accelerate HIV prevention and help the world achieve epidemic control. Navigating these seismic developments requires unprecedented coordination, solidarity, and courage.
Global health champions can defy the hatred, fear, and greed that are dominating politics in so many places around the world. Together we can innovate, create, and protect the advance of HIV prevention and global health. This issue provides a snapshot on threats to delivering PrEP, the potential of injectable lenacapavir (LEN) for PrEP, and on the implications of upstream research and development of other long-acting PrEP.
Read below or download the PDF version.
Progress in PrEP Uptake: Threatened
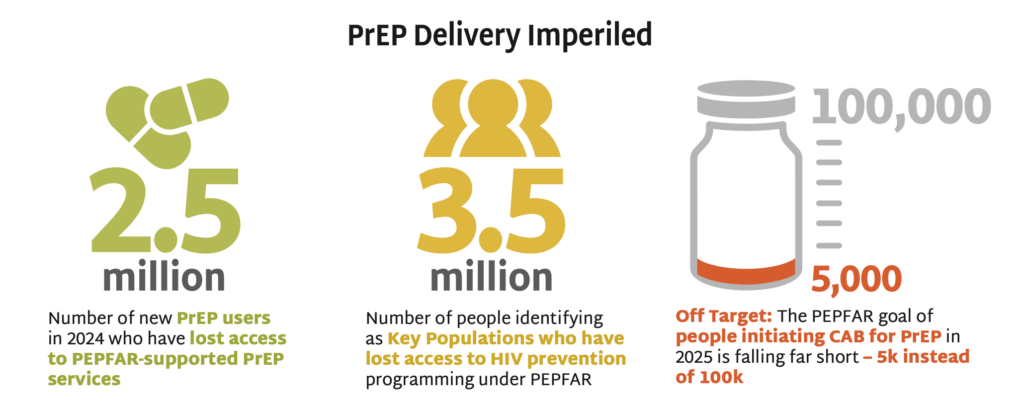
- PEPFAR documented 2.5 million new PrEP users in 2024, who could now lose access to PEPFAR- supported PrEP services. US Department of State issued a limited and inconsistently implemented waiver in February, allowing for continued provision of HIV treatment but restricting PrEP access to pregnant and lactating people only.
- These actions will result in 3.5 million who identify as key populations (KPs) losing access to all HIV prevention programming under PEPFAR, according to 2024 PEPFAR data tracking KP use of PrEP. These groups have higher rates of HIV incidence and face additional barriers to accessing services now that targeted programs are suspended.
- PEPFAR had a goal of 100,000 people initiating cabotegravir (CAB) for PrEP in 2025. But only 5,000 individuals had initiated by October 2024. The suspension of PEPFAR funding imperils scale-up of this long-acting product.
- These figures represent just some of the disruptions that are decimating PrEP delivery. Learn more here: Impact of PEPFAR Stop Work Orders on PrEP
- Overcoming this challenge, restoring, sustaining, and accelerating PrEP access is imperative and possible if the field works together.

For the last eight years, AVAC has proudly worked with PEPFAR to document PrEP uptake and its impact around the world. That stopped in January with a stop work order from the US government. But protecting access to PrEP is vital. Are you leading a PrEP program? Whether supported by PEPFAR or not, we invite you to work with us to ensure global data on PrEP is not lost. Find us at [email protected].
PrEParing for New Products
- Approval by the US Food and Drug Administration (FDA) for injectable 6-month LEN for PrEP is expected in June, with WHO guidelines expected in July. See the full timeline.
- Modelling data from South Africa demonstrate the potential of injectable PrEP to dramatically reduce HIV incidence by up to 90% by 2044, and potentially even sooner with more aggressive uptake. This potential goes beyond South Africa, lighting the way toward epidemic control the world over.
- The field must be prepared for swift action once LEN is approved and recommended, to ensure this opportunity is not squandered. As AVAC’s interactive timeline, Tracking LEN Rollout, outlines, donors, ministries of health, manufacturers, regulators, and civil society all have a role to play to pave the way for swift, equitable and effective introduction of LEN for PrEP.
The Latest R&D in the Prevention Pipeline
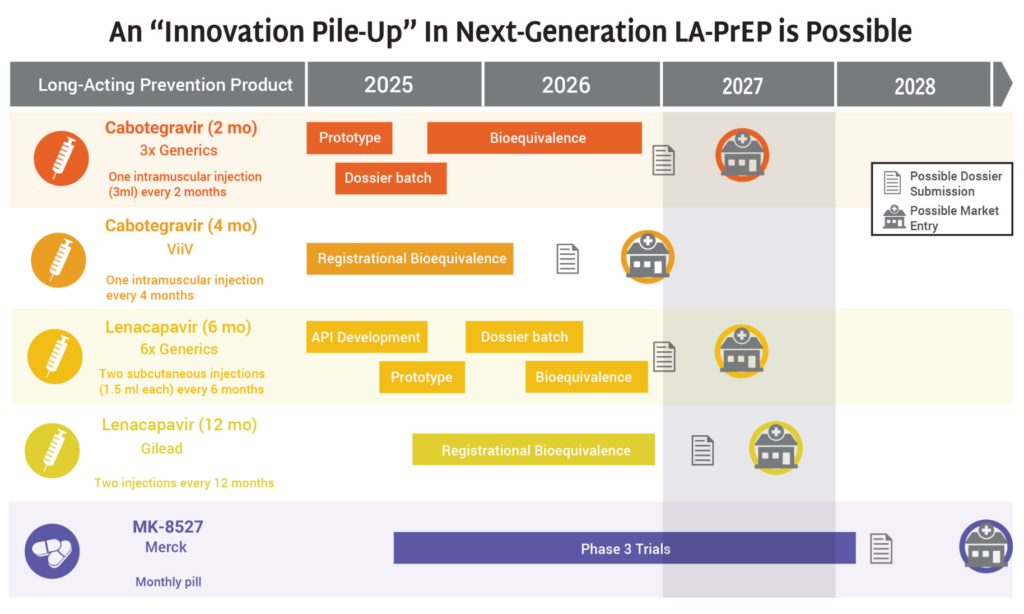
- The HIV prevention market is headed toward a period of significant opportunity—and possible congestion—as a slate of new products are on track for continued development and potential introduction to the market in 2027 and 2028.
- Generics for 2-month CAB and 6-month LEN, along with ViiV’s 4-month CAB, Gilead’s 12-month LEN, and Merck’s monthly oral MK-8527 PrEP pill (if further development and approvals are successful) could all enter the market by 2028.
- The possibility of so many products on the market, including four different formulations of injectable PrEP, means that it is imperative the field prepares for this future now.
- Markets and policies must be built to support the products in the market already, so that new options can be rapidly deployed and deliver impact. Otherwise, the field will squander time and money, with epidemic control slipping further out of reach.
- With US funding cuts to both HIV prevention R&D and delivery, communities must be engaged, supported, and informed about all prevention options, and the choices that all stakeholders will need to make. This means gathering and sharing data and information about cost-effectiveness, user acceptability, program feasibility, and impact. Communities empowered with the facts can advocate for the choices they need, and push ministries of health to make strategic investments and procure the prevention method mix that fits their context and delivers impact.
Prevention Playlist
AVAC develops a wide range of resources to inform decision making and action. Check out the latest:
Join
- Subscribe to Global Health Watch: AVAC’s weekly newsletter dedicated to breaking down critical developments in US policies and their impact on global health, at avac.org/global-health-watch
- CHANGE: In response to the unfolding crisis, more than 1,500 people from civil society organizations around the world have launched CHANGE—Community Health & HIV Advocate Navigating Global Emergencies—a coalition formed to support urgent action: [email protected]
Use
- Research Matters Advocacy Toolkit, AVAC
- Tracking the Freeze: Real-Time Impact on Key Populations, GBGMC
- Impact of the Stop-Work Order on PrEP, AVAC
- Tracking Lenacapavir Rollout, AVAC
- PEPFAR Program Impact Tracker, Impact Counter
- Weekly Situation Report, UNAIDS
Watch/Listen
- Politics and Global Health: The Need for a New, Resilient Architecture, Webinar
- Lawsuit Wins and What’s at Stake: AVAC v US Department of State, PxPulse episode
- Global Health in the Lurch: What’s happening now and who is pushing back, PxPulse episode
- Advancing Sexual and Reproductive Health and Rights: Moving forward post-the 2024 US Election, Webinar
Read
- Despite USG Global Health Collapse, Here Are Several Data Trackers To Support Your Advocacy, AVAC
- On Going Backwards, Lancet
- The Trump Administration’s Foreign Aid Review: Status of PEPFAR, KFF
- The Best Investment You Didn’t Know You Made: How NIH Funding Fuels Innovation and Economic Growth, amfAR
- HIV Market Impact Memo, CHAI
- The USAID List of Terminated Global Health Awards – What Does it Tell Us?, KFF
- AVAC Condemns HHS Mass Layoffs
- PrEP in the Balance: Hopes and fears in 2025
- Better Engagement, Better Evidence: Working in partnership with patients, the public, and communities in clinical trials with involvement in good participatory practice, Lancet
- PEPFAR: A Strategic Necessity for US Leadership and Global Health, AVAC
- Rallying for HIV Prevention Amid Policy Attacks, AVAC
- CROI 2025 Shows the Promise of Research at its Best, AVAC
Impact of PEPFAR’s Stop Work Order on PrEP
The impact of the stop-work order on PrEP is expected to be severe. In a set of slides and on our website, PrEPWatch, we have posted the results of an analysis drawing on key informant interviews with representatives of Ministries of Health and PrEP implementers between 27 January 2025, when stop-work orders were issued by the US government, and the end of February 2025, when the vast majority of USAID-funded projects received official termination notices. Find the latest here.
Trials & Projects Halted by USAID Funding Suspension
The stop-work orders have disrupted USAID-supported HIV prevention research, halting critical investigations in vaccine and next-generation PrEP strategies.
The abrupt suspension of these trials also raises serious ethical concerns. Stopping trials mid-course undermines trust in research, jeopardizes community engagement, and abandons participants who volunteer their bodies for scientific discovery. It will take years to build back this critical infrastructure—for HIV research and beyond—as well as the community partnership and trust needed to ensure smooth and ethical research.
HIV Incidence, Age 15-49
Looking backward and then into the future, this chart shows actual HIV rates alongside projected rates with and without current prevention strategies (PrEP, VMMC, and free condoms).
Global PrEP Uptake and PEPFAR’s Role, 2016-2024
AVAC’s Global PrEP Tracker has documented cumulative PrEP initiations on a quarterly basis for nearly a decade. This graph presents the final data collected while PEPFAR was fully operational—PEPFAR support was responsible for 79% of PrEP uptake globally in the last year and reached 83% by the end of September of 2024. Data on the fourth quarter of 2024 is inaccessible since PEPFAR was taken offline in late January.
PxWire Volume 15, Issue No. 1
In this special edition of Px Wire, AVAC is going beyond a quarterly update of biomedical HIV prevention. In this issue, we look at how the new US Administration’s attack on global health can be expected to devastate HIV prevention, including the capacity to deliver existing PrEP options, the scale up of new PrEP products, and the paralyzing impact on research and development. A PDF version of this report is also available.
From Research to Rollout: The impact of USG global health pullout


The United States’ presidential regime has launched a sustained, multi-pronged attack against foreign assistance, scientific inquiry, due process and good governance. It threatens economies, human rights, international partnerships, global health at large, and the rule of law. For HIV prevention, a single sentence, issued in a February 6 advisory from the US Department of State, has derailed the entire field, potentially setting back the HIV response by years, if not decades. Read on for resources to support your advocacy and fortify our solidarity at this critical time.
Progress in PrEP Uptake: Threatened
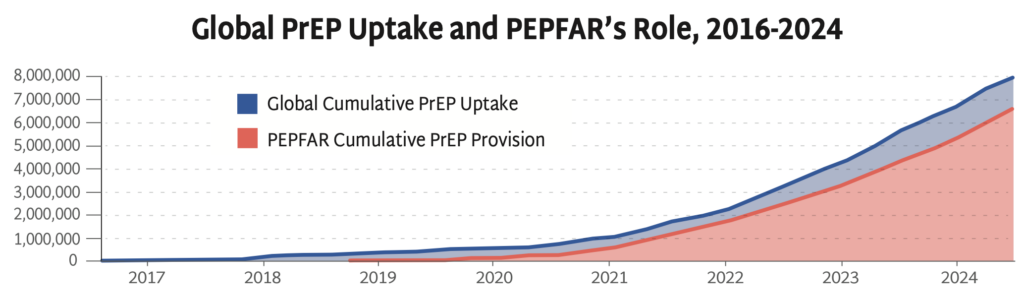
PEPFAR has been pivotal to accelerating PrEP uptake, significantly expanding HIV prevention coverage. The freeze on foreign aid prohibits funding to PEPFAR’s PrEP programs and poses a serious threat to global efforts to control the epidemic.
AVAC’s Global PrEP Tracker has documented cumulative PrEP initiations on a quarterly basis for nearly a decade. This graph presents the final data collected while PEPFAR was fully operational—PEPFAR support was responsible for 79% of PrEP uptake globally in the last year and reached 83% by the end of September of 2024. Data on the fourth quarter of 2024 is inaccessible since PEPFAR was taken offline in late January.
At the time of the foreign aid freeze, PrEP uptake had reached 8 million initiations since 2016, an achievement that’s taken almost 10 years to reach—too slow and too small to reach UNAIDS targets, but a robust foundation to finally accelerate PrEP uptake with next-generation PrEP. Current US policies, instead of expanding PrEP coverage, are leading to the closure of programs, and will reverse global progress against HIV.
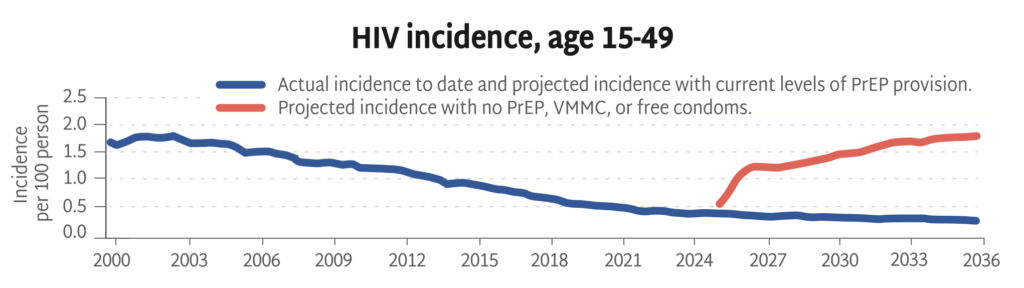
Without primary prevention, the HIV epidemic is poised to rage on, with incidence among adults on track to triple over the next ten years. This HIV Synthesis model, developed by the HIV Modelling Consortium, estimates the impact of stopping all HIV prevention services across Africa from now through 2036—including PrEP, voluntary medical male circumcision (VMMC), and free condom distribution.

For the last 8 years, AVAC has proudly worked with PEPFAR to document PrEP uptake and its impact around the world. That stopped in January with a stop work order from the US government. But protecting access to PrEP is vital. Are you leading a PrEP program? Whether supported by PEPFAR or not, we invite you to work with us to ensure global data on PrEP is not lost. Find us at [email protected].
PrEParing for New Products: Is rollout still possible?

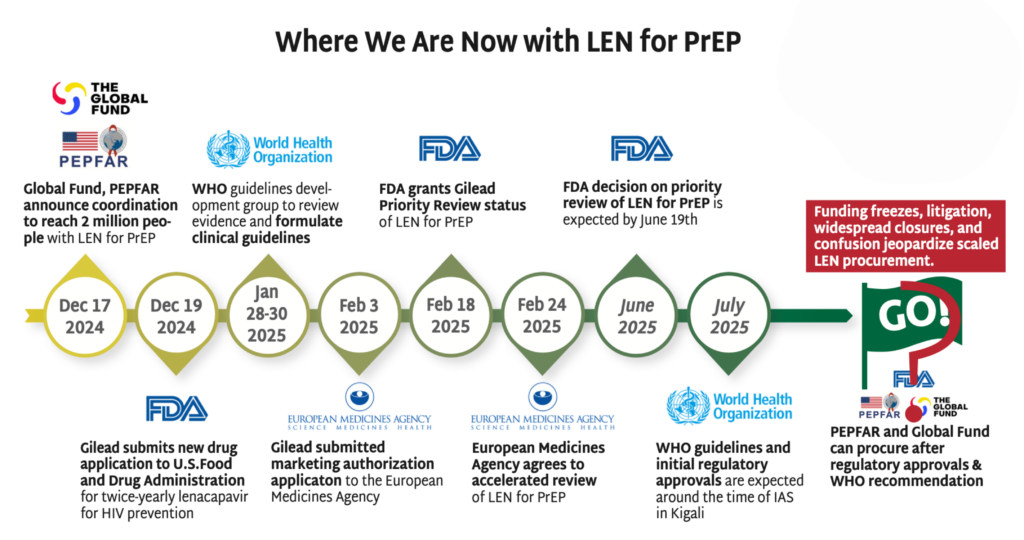
Read more in The Gears of Lenacapavir for PrEP Rollout.
The chaos in foreign assistance programs (including discontinuation of major PrEP programs), cuts in staffing and new demands on donor commitments will make decisions on the procurement of LEN for PrEP more complex and uncertain. In December 2024, the Global Fund and PEPFAR announced a plan to reach 2 million people with LEN for PrEP over three years. Exactly how funding to support this unprecedented introduction program will move forward, in the absence of significant US investment, is far from certain. The other stakeholders, including Global Fund, Gilead, CIFF and the Gates Foundation expressed commitments to the deal, but major questions remain. In the meantime:
- Gilead’s production of LEN for PrEP is continuing, as is the technology transfer to generic license holders.
- The FDA granted Gilead priority review status for LEN for PrEP, with a decision due by June 19, 2025.
- The EMA has agreed to an accelerated review of LEN for PrEP for both European access and as part of the EU-Medicines for all (EU-M4all) program, reducing the review from seven months to five months.
- The WHO’s Guideline Development Group (GDG) met in January, and WHO is expected to issue guidelines by July 2025.
The Latest R&D in the Prevention Pipeline: Supported or undermined?

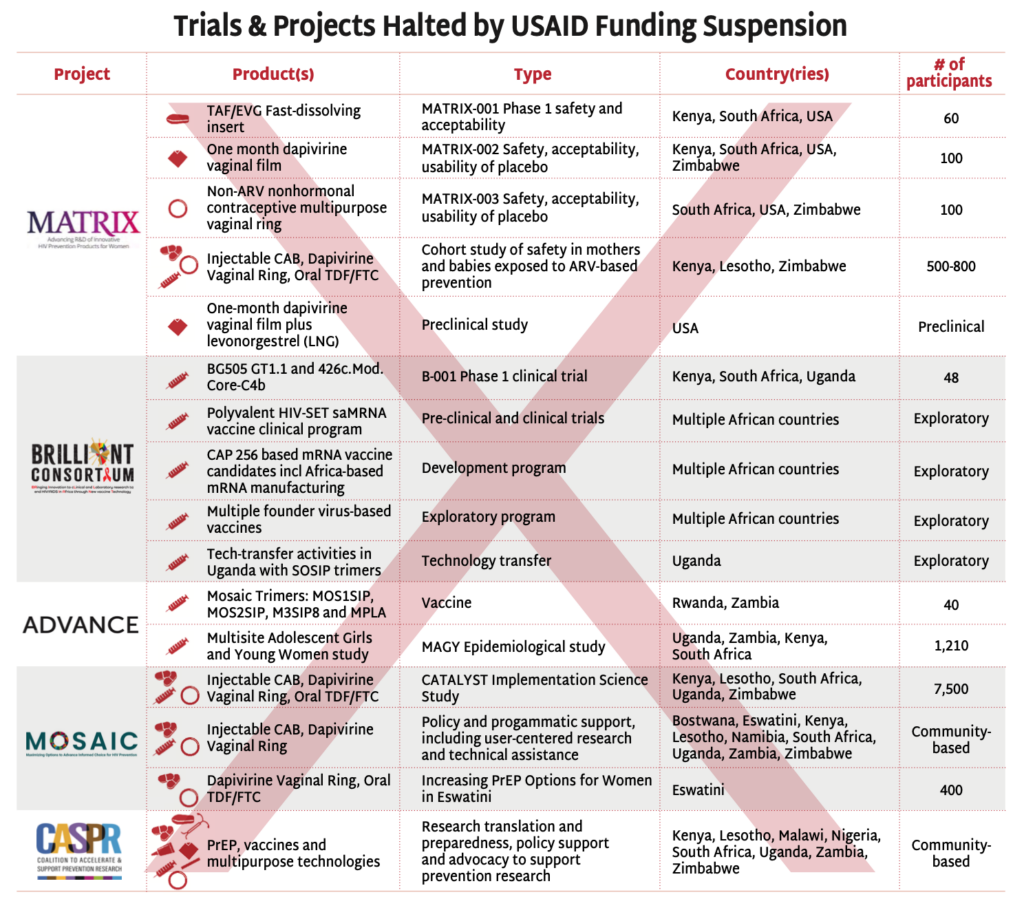
The stop-work orders have disrupted USAID-supported HIV prevention research, halting critical investigations in vaccine and next-generation PrEP strategies.
- The BRILLIANT and ADVANCE projects’ clinical, preclinical, and experimental trials testing HIV vaccine candidates have been suspended.
- The MATRIX projects’ driving innovation with next-generation PrEP and MPT products, fast-dissolving inserts and vaginal films and rings, have been forced to stop their clinical trials.
- The MOSAIC projects’ have suspended all implementation science activities, including the CATALYST study, investigating choice among oral PrEP, injectable cabotegravir and the dapivirine vaginal ring. Other implementation studies are continuing, but access to the commodities, much of which was procured by PEPFAR is questionable. See AVAC’s Integrated Study Dashboard for details.
- The Coalition to Accelerate and Support Prevention Research (CASPR) has also been paused. Led by AVAC in partnership with a number of leading African civil society organization, CASPR focuses on building an enabling environment for HIV prevention R&D. (Note: In early February, AVAC lead a lawsuit against the State Department seeking emergency relief from the freeze on foreign assistance, including funding for CASPR. The case, AVAC v. United States Department of State, is pending.)
These disruptions delay the development of urgently needed HIV interventions and threaten the sustainability of research infrastructure all over the world, with particularly egregious impacts on the research capacity of regions most impacted by the epidemic.
The abrupt suspension of these trials also raises serious ethical concerns. Stopping trials mid-course undermines trust in research, jeopardizes community engagement, and abandons participants who volunteer their bodies for scientific discovery. It will take years to build back this critical infrastructure—for HIV research and beyond—as well as the community partnership and trust needed to ensure smooth and ethical research.
Prevention Playlist
AVAC develops a wide range of resources to inform decision making and action. Check out the latest:
Join
- Subscribe to Global Health Watch: AVAC’s weekly newsletter dedicated to breaking down critical developments in US policies and their impact on global health. avac.org/signup
- Seeking Visuals and Videos: Leading groups in Washington, DC, are urgently trying to collect videos and photos documenting the impact of the US government’s foreign aid freeze, such as clinic closures despite the waiver. Non-professional phone videos and photos are welcome. Send to [email protected] for more details
- CHANGE: In response to the unfolding crisis, more than 1,300 people from civil society organizations around the world have launched CHANGE—Community Health & HIV Advocate Navigating Global Emergencies—a coalition formed to support urgent action. [email protected]
Use
- Graphic of studies of injectable cabotegravir and the dapivirine vaginal ring in eastern and southern Africa, AVAC
- Most Lifesaving Services Remain Paused: A Rapid Assessment of the PEPFAR Stop Work Order, amfAR, CHANGE, Data ETC
- What Effect are HIV Programmes Having in Africa, The HIV Modelling Consortium
- PEPFAR & Global Fund Support for HIV Programs, amfAR & Data ETC
Watch/Listen
- [LISTEN] Would PEPFAR Survive Trump—and what would it look like?
- The Impact and Implications of Recent US Government Federal Funding Reductions on Health Programmes, The Steve Biko Centre for Bioethics at The University of the Witwatersrand event recording
Read
- AVAC v United States Department of State. On February 10, 2025, AVAC and another nonprofit organization sued the new US Administration, seeking emergency relief from an Executive Order that inhumanely froze all funding for foreign assistance, AVAC
The Human Cost of PEPFAR’s PrEP Restrictions
The President’s Emergency Plan for AIDS Relief (PEPFAR) has long been hailed as one of the most successful and bipartisan efforts in global health. Established in 2003 under the Bush Administration, PEPFAR has saved millions of lives by providing critical HIV treatment and prevention services and building partnerships with countries and communities. But this work ground to halt last week, with a chilling pause on all work.
This week, the State Department approved a limited waiver to re-start some treatment and PMTCT programs is a small step forward, but far from a victory at all. And it is especially short-sighted and cruel in it approach (or lack thereof) to primary HIV prevention. One of the most effective tools in the fight against HIV has been Pre-Exposure Prophylaxis (PrEP), a medication regimen that reduces the risk of acquiring HIV by over 99% when taken consistently. Yet, with this new guidance, the Trump Administration is choosing politics over science, discrimination over compassion, and ultimately, death over life.
The February 6th guidance from the Trump Administration stating that “people other than pregnant and breastfeeding women who may be at high risk of HIV infection or were previously initiated on a PrEP option cannot be offered PEPFAR-funded PrEP during this pause of US Foreign Assistance or until further notice” is not only a dangerous deviation from sound public health policy—it is a death sentence for thousands of people at risk of HIV globally.
PrEP is one of the most powerful tools available in the fight against HIV/AIDS. Public health experts and epidemiologists agree that expanding access to PrEP is essential to curbing new infections. The administration’s directive effectively shuts the door on communities around the world, depriving them of life-saving medication and increasing the risk of new HIV transmissions. This move not only contradicts decades of scientific research but also undermines the very mission of PEPFAR: to save lives and reduce the burden of HIV/AIDS worldwide.
The restriction on PrEP access is particularly troubling in regions where the HIV epidemic is most severe, such as sub-Saharan Africa. In these areas, young women account for a disproportionate number of new infections, but so do men who have sex with men (MSM) and transgender individuals—groups that are now explicitly excluded from PEPFAR-funded PrEP under this new policy. By denying these populations access to PrEP, the administration is actively allowing the HIV epidemic to spiral further out of control.
Research has continued to produce newer modalities of PrEP that are long-acting products, which create even more possibilities for people to protect themselves for up to six months per dose. One long-acting prevention tool, injectable lenacapavir, is currently under FDA review and could provide a valuable option for people who have trouble with daily pill-taking or fear the stigma that is sometimes associated with ARVs in their communities. With regulatory approval and WHO guidelines expected by the middle of the year, injectable lenacapavir provides the best chance to drive down the number of new infections. So stopping current PrEP programs makes seizing this new opportunity that much harder.
This decision appears to be less about public health and more about an ideological agenda that seeks to police morality rather than protect lives. The new Trump Administration, just weeks into its second term is demonstrating a careless pattern of undermining global health programs, including cutting funding for international health organizations that provided comprehensive sexual health services. This latest move is yet another example of the administration prioritizing conservative politics over the well-being of vulnerable populations.
By selectively restricting PrEP access to only pregnant and breastfeeding women, the administration is effectively signaling that only certain groups are deemed “worthy” of HIV prevention. This echoes the stigmatizing rhetoric that has long plagued HIV/AIDS policy, one that associates the disease with so-called “immoral” behavior and seeks to punish those who are at highest risk. Such policies not only fail to address the reality of the HIV epidemic but also reinforce dangerous stereotypes that fuel discrimination and stigma.
The repercussions of this policy extend far beyond the immediate communities affected. As the largest funder of global HIV/AIDS programs, the United States has a moral and strategic responsibility—and opportunity—to lead with science and evidence-based solutions. The decision to restrict PrEP access will not only increase new infections but also put added strain on already overburdened healthcare systems. The cost of treating HIV is significantly higher than preventing it, making this policy both a moral and fiscal failure.
Moreover, at a time when the world is grappling with multiple global health crises, the US should be strengthening, not weakening, its commitment to international health initiatives. This policy shift undermines trust in US global health leadership and sends a dangerous message to other nations that discrimination and exclusion are acceptable public health strategies.
Congress, global health advocates, and the public must demand the immediate reversal of this harmful policy.
The fight against HIV/AIDS is far from over. We cannot afford to take steps backward when so many lives are at stake. The Trump Administration’s decision to deny PrEP to those most at risk is a dereliction of duty, a moral failing, and a betrayal of the very principles that PEPFAR was founded upon. The world is watching, and history will not judge kindly those who choose exclusion over compassion, politics over science, and death over life.
Gears of Lenacapavir for PrEP Rollout
The Gears of Lenacapavir for PrEP Rollout outlines a transformative opportunity in the global fight against HIV, coming at a pivotal moment when the scale-up of PrEP has shown remarkable progress but remains insufficient to achieve a transformational impact on HIV incidence and the trajectory of the epidemic. This effort demands a coordinated response from governments, donors, civil society, and the private sector to ensure rapid implementation, equitable access, and sustainable impact. By leveraging lessons from previous PrEP interventions, aligning financing mechanisms, and prioritizing underrepresented regions, stakeholders can overcome systemic barriers and maximize the public health potential of this innovative long-acting prevention tool.
With anticipated regulatory approvals and production scaling, the plan targets over 2.5 million LEN users in low- and middle-income countries by 2027. With a focus on addressing structural barriers like stigma, healthcare inequities, and restrictive policies, alongside integrating generics into national programs, the roadmap aims to build on existing progress while accelerating the pace of HIV prevention.