This issue showcases the status of access to oral PrEP with the best data available since the US foreign aid freeze disrupted PEPFAR operations beginning in January 2025. Stakeholders are digging deep to meet the moment, which includes an unprecedented opportunity to drive down HIV incidence with the rollout of injectable lenacapavir (LEN) for PrEP.
A new AVAC infographic below depicts the accelerated process for delivering LEN to date, and critical next steps. Lastly, our update on the HIV vaccine R&D pipeline documents an evolving and diverse portfolio of products, which remains essential for a durable end to HIV as a global health threat.
Read below or download a PDF version of this issue.
Progress in PrEP Uptake

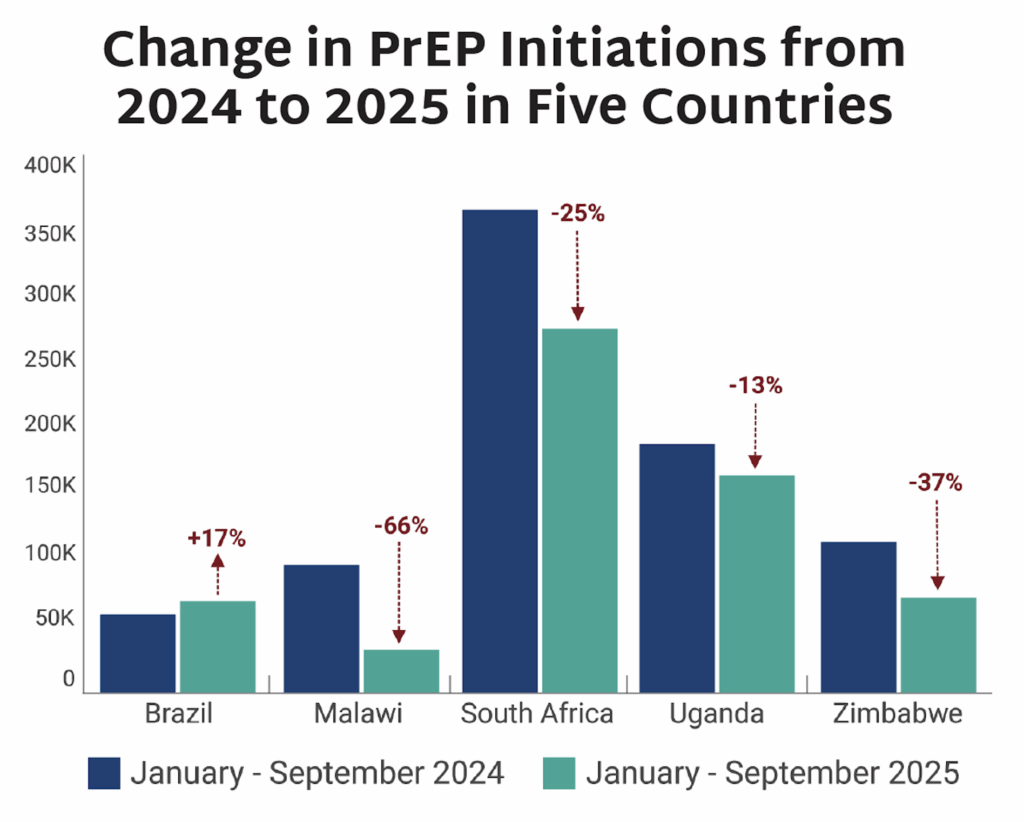
- Between the period January-September 2024 to January-September 2025, PrEP initiations fell between 13% and 66% in selected high-volume PrEP countries where ministries of health (MoH) were able to provide data.
- Among the five countries depicted, four saw significant decline in PrEP uptake. All four relied on PEPFAR PrEP programs and were disrupted by stop work orders (SWO).
- Brazil does not receive PEPFAR funding for PrEP and was the only country surveyed that saw an increase in initiations, up by 17%.
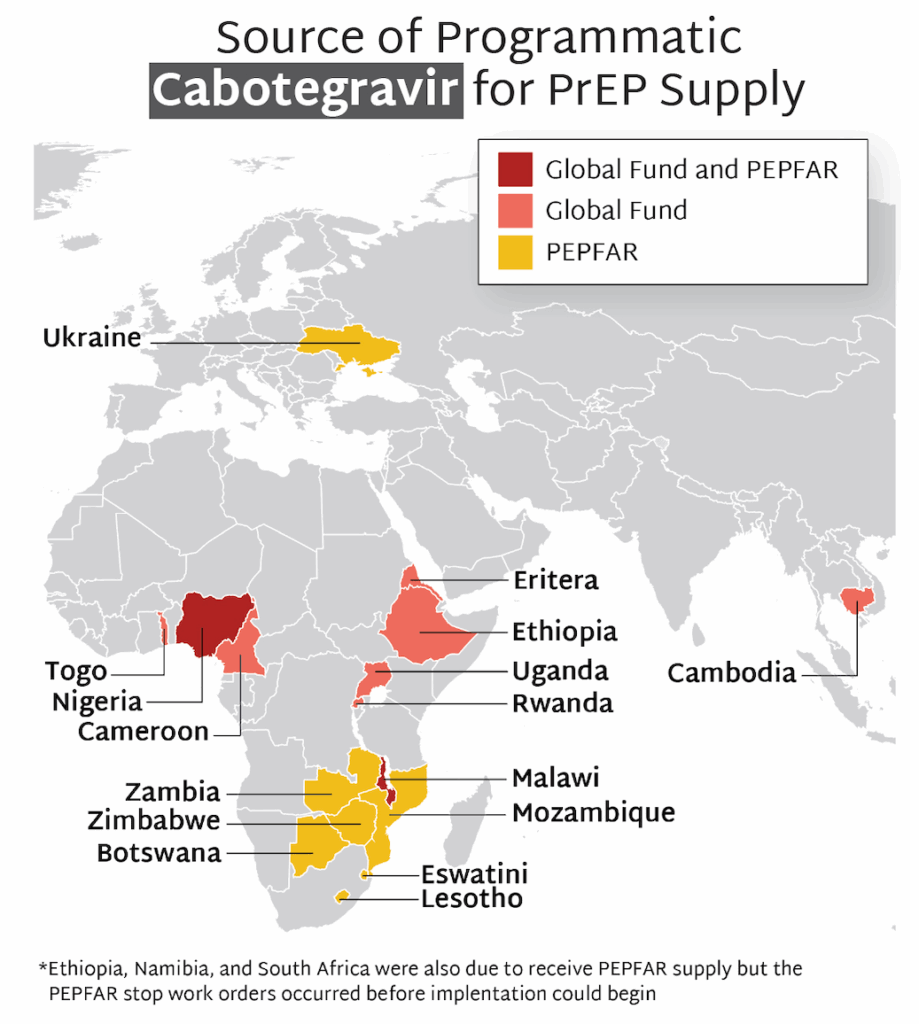
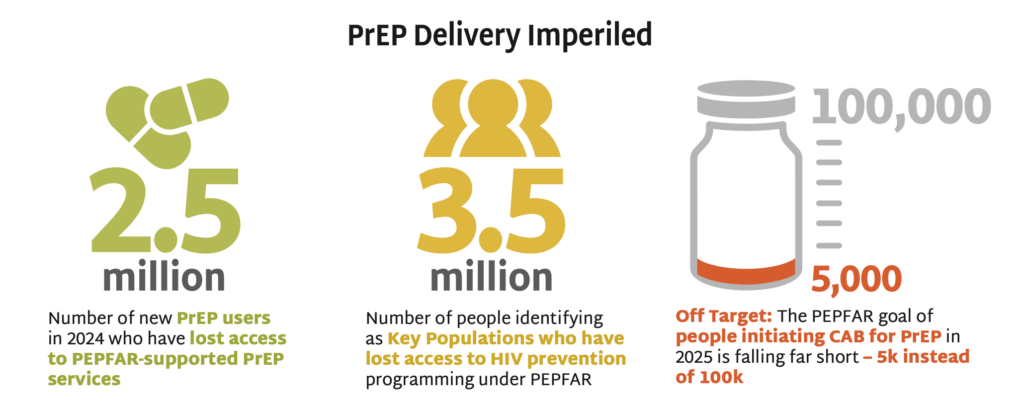
- Among the same four African countries, data on initiations of long-acting injectable cabotegravir (CAB) for PrEP show a combined total of 5,709 initiations during this period. With PEPFAR’s original target of 100,000 initiations across ten African countries by the end of 2025, these data reflect an extreme shortfall of CAB uptake, caused by PEPFAR’s discontinuation of CAB procurement and programs.
- A decline in PrEP uptake represents a serious threat to HIV prevention and to efforts to achieve epidemic control. A recent analysis in The Lancet HIV estimated that stopping PEPFAR’s PrEP programs in Africa for a year could lead to approximately 7,000 additional new HIV acquisitions, and more than 10,000 additional cases of secondary transmission in the next five years. This slowdown also makes the introduction of new options more challenging.
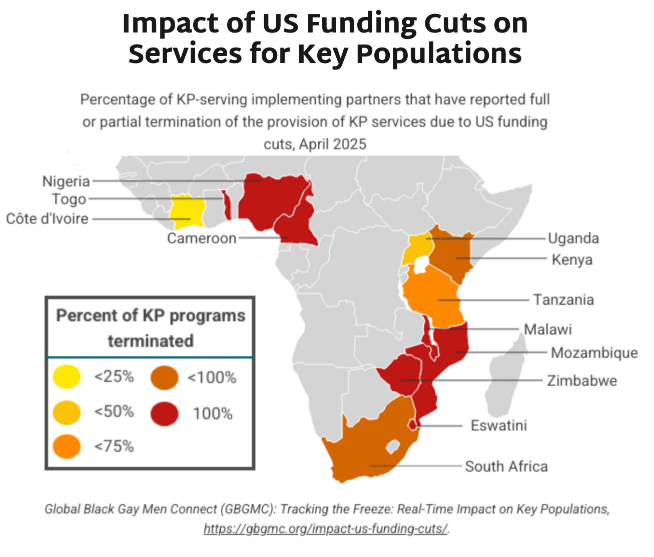
- For more details on the negative impact of the PEPFAR SWO, including mitigation strategies that ministries of health and program implementers are putting in place, see our SWO Tracker.
PrEParing for New Products

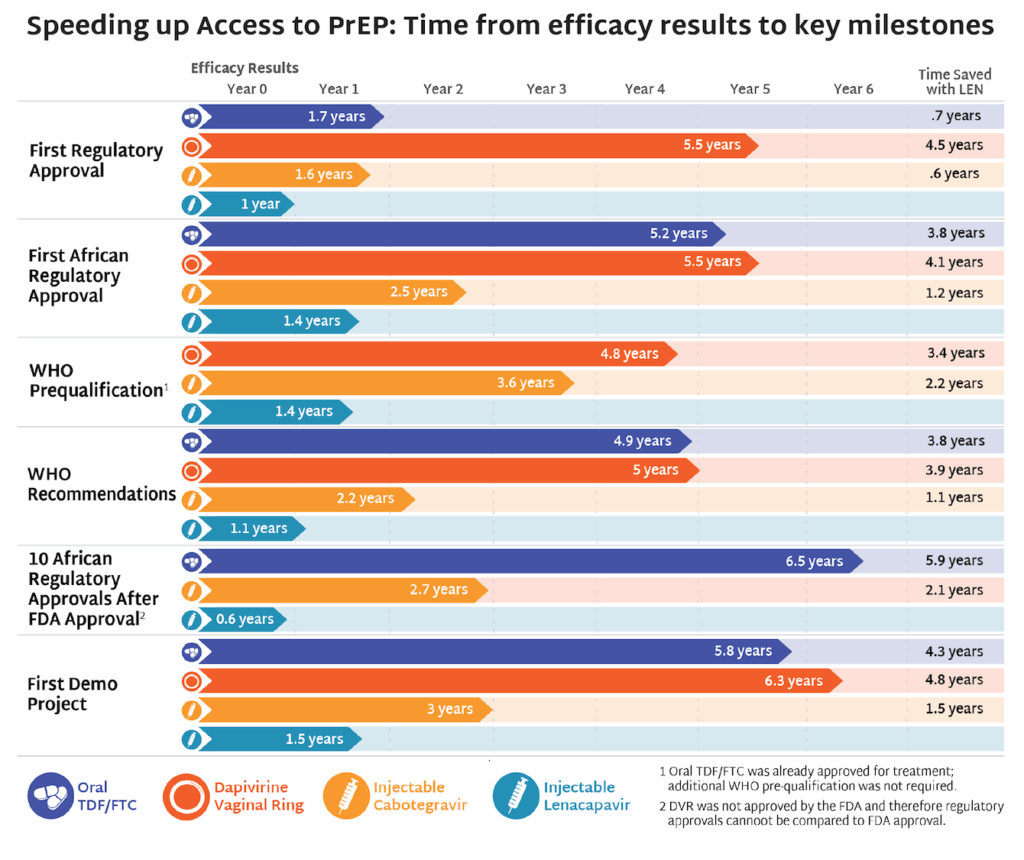
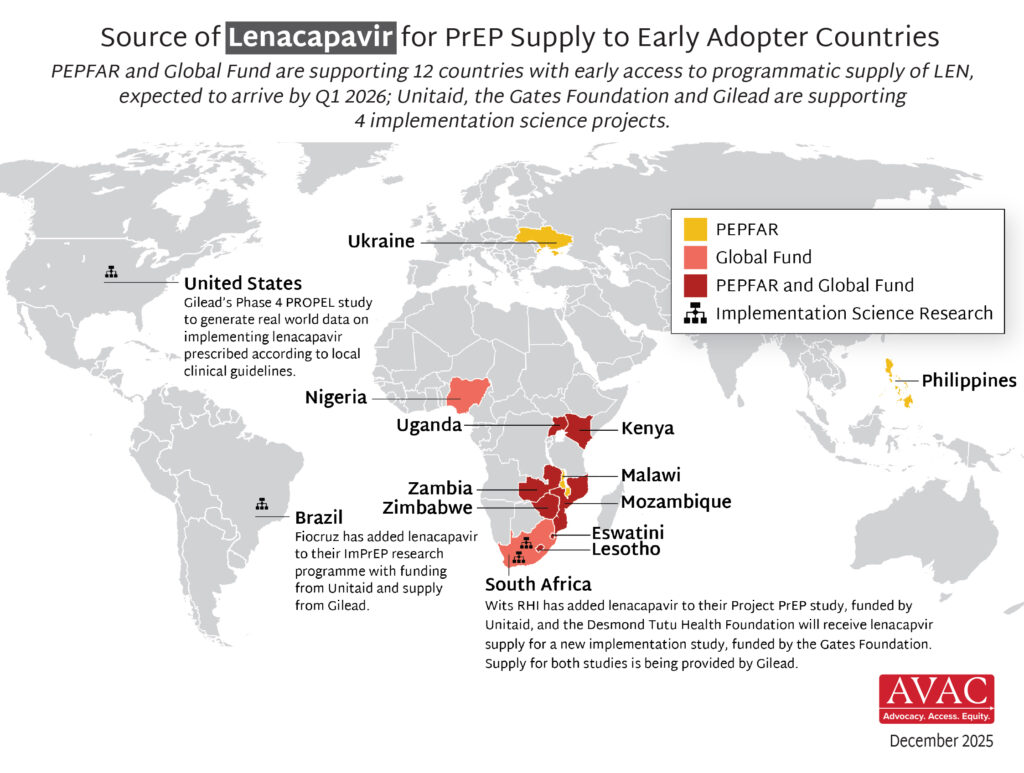
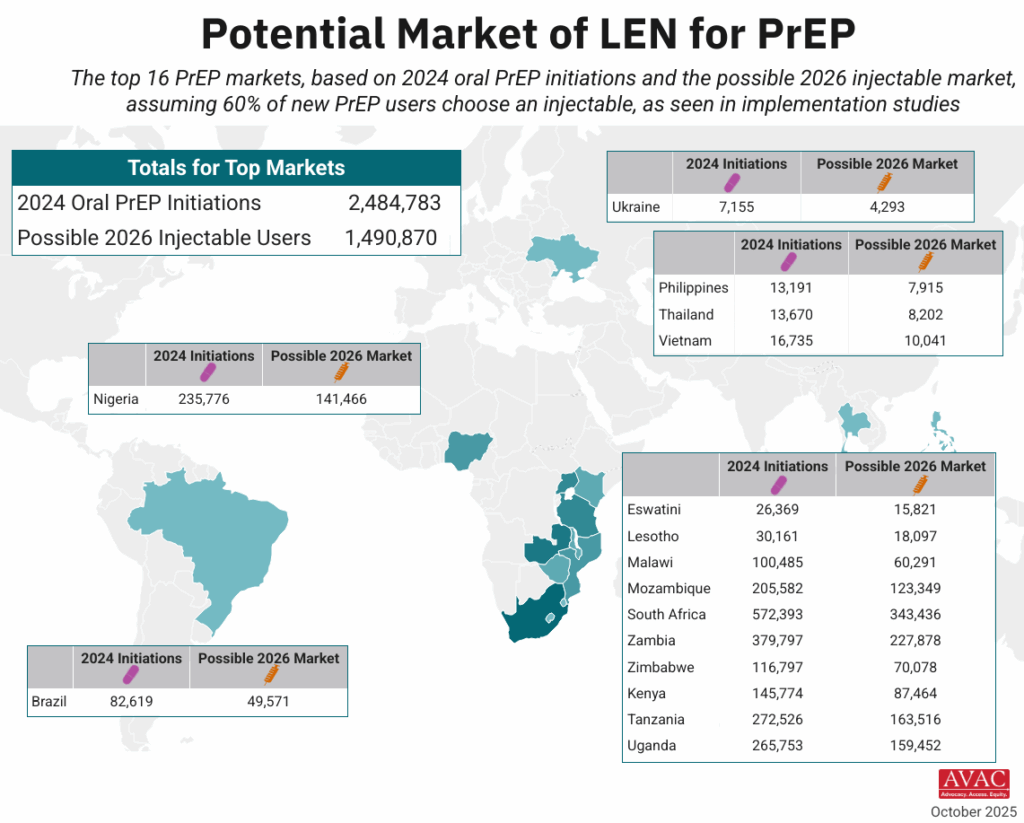
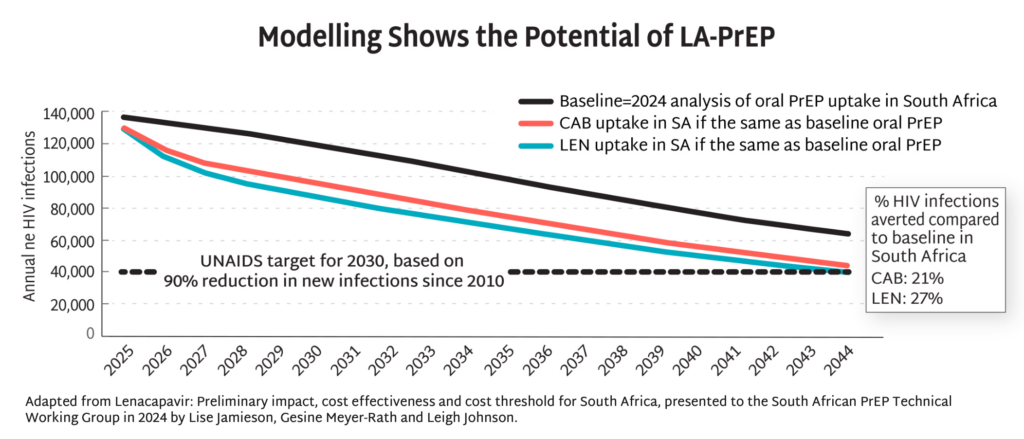
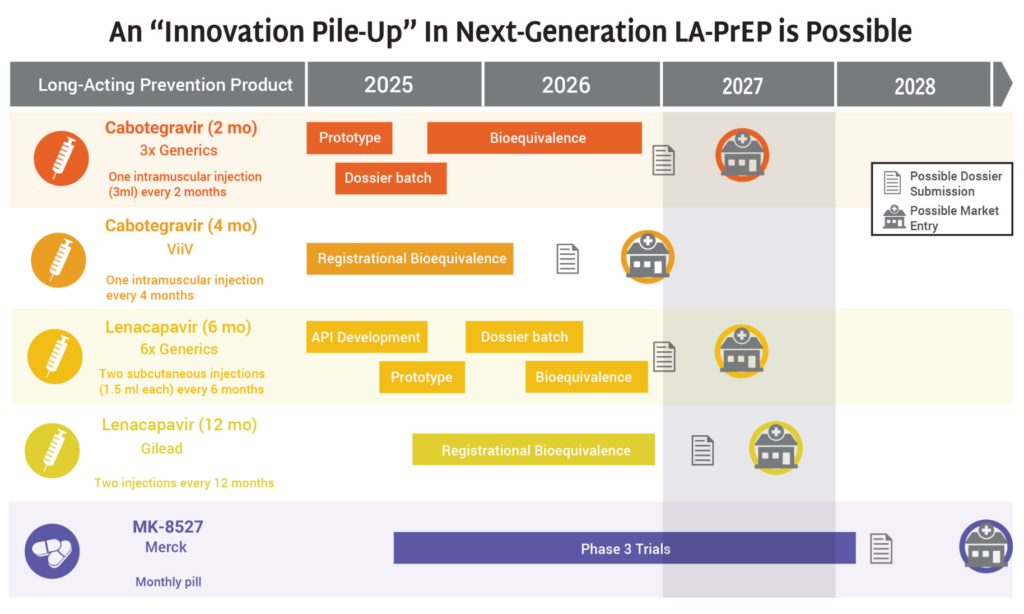
- The timeline for hitting key milestones in product introduction is moving faster for injectable LEN than for any previous PrEP products, starting from the announcement of efficacy results in Phase III trials.
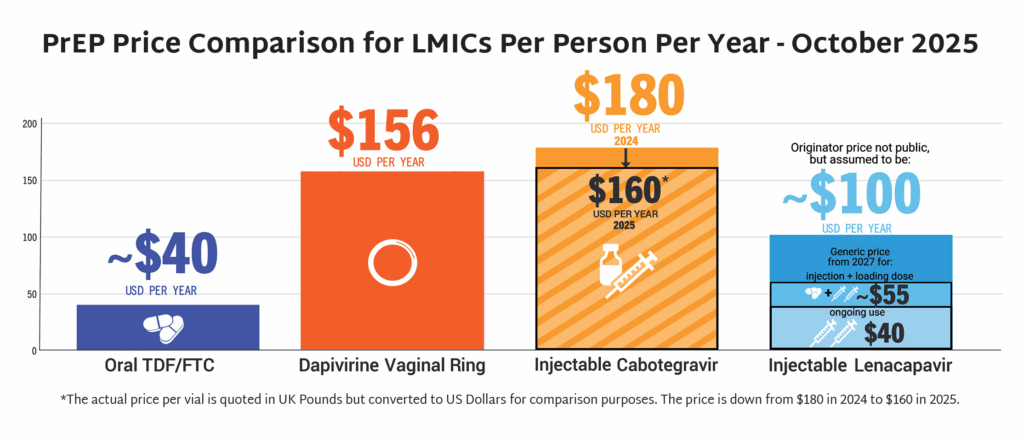
- LEN’s accelerated timeline compared to oral PrEP, DVR, and CAB reflects a field-wide effort to learn lessons from previous PrEP rollout and not repeat the mistakes of the past.
- The developers of LEN, Gilead Sciences, leveraged the EU-M4all process, formerly known as Article 58, and WHO’s Collaborative Registration Procedure (CRP) to speed the process of regulatory review in low-and middle-income (LMIC) countries. This process allows the European Medicines Agency (EMA) and the WHO to provide a scientific opinion on products that address “diseases of major public health interest” in LMICs. EU-M4all and CRP help national regulators accelerate their review.
- LEN has received 10 African regulatory approvals in only six months since the US Food and Drug Administration granted approval. By comparison, oral PrEP took 3.5 years for the first African approval to be granted after FDA approval, and 6.5 years to reach 10 approvals.
The Latest R&D in the Prevention Pipeline

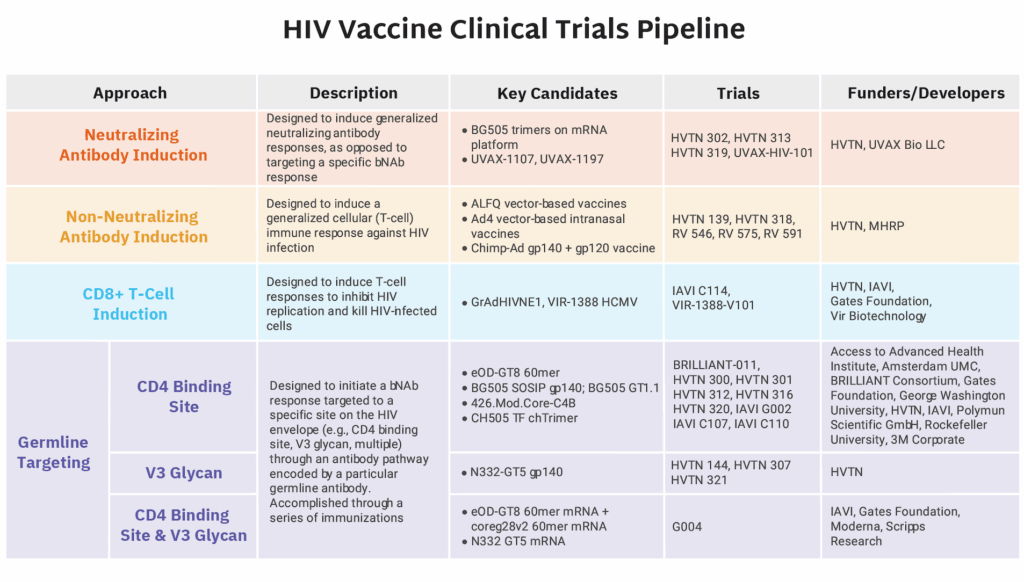
Despite the challenges posed for research in 2025, and HIV research in particular, two notable vaccine trials launched at the start of 2026:
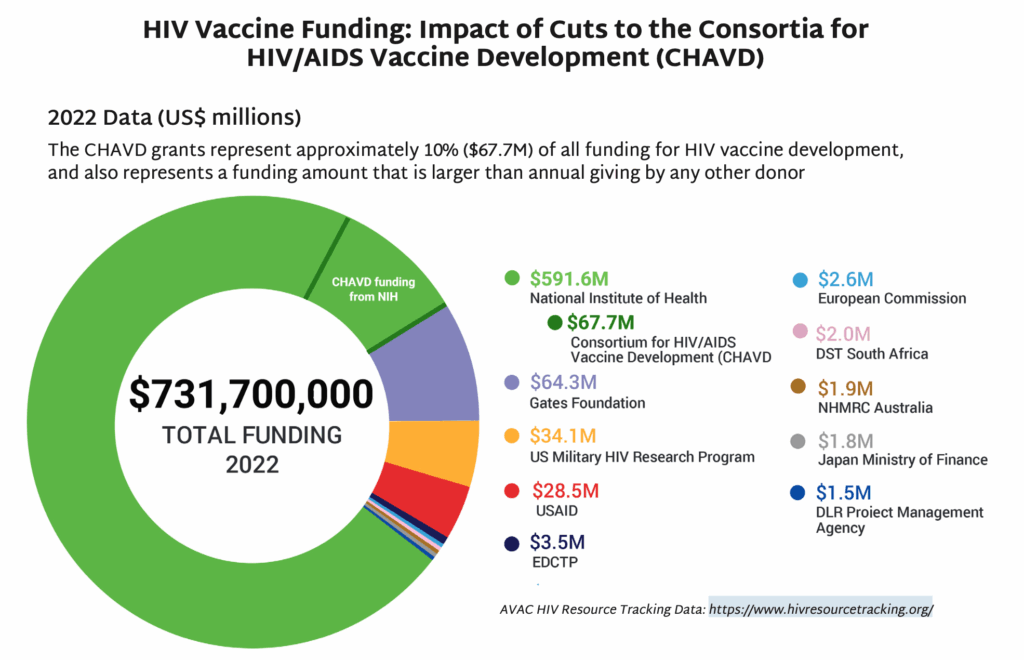
- BRILLIANT 011 launched in South Africa. This HIV vaccine trial was cancelled by the US government as part of the dismantling of USAID in early 2025. The South African Medical Research Council and the Gates Foundation, however, provided fund-ing to keep the program going as a smaller, more streamlined version of the trial originally planned under USAID. This groundbreaking African-led and -funded trial is a Phase I study of a bNAb inducing candidate. Learn more about vaccine strategies here.
- The first vaccinations for the IAVI G004 Phase I clinical trial also took place in January. This early-stage HIV vaccine trial stands out as the first germline-targeting vaccine designed to induce broadly neutralizing antibodies (bNAbs) against more than one major site on the HIV envelope—specifically both the CD4 binding site and the V3 glycan region. Most prior HIV vaccine candidates have focused on the CD4 binding site alone.
Additional developments in the R&D pipeline include:
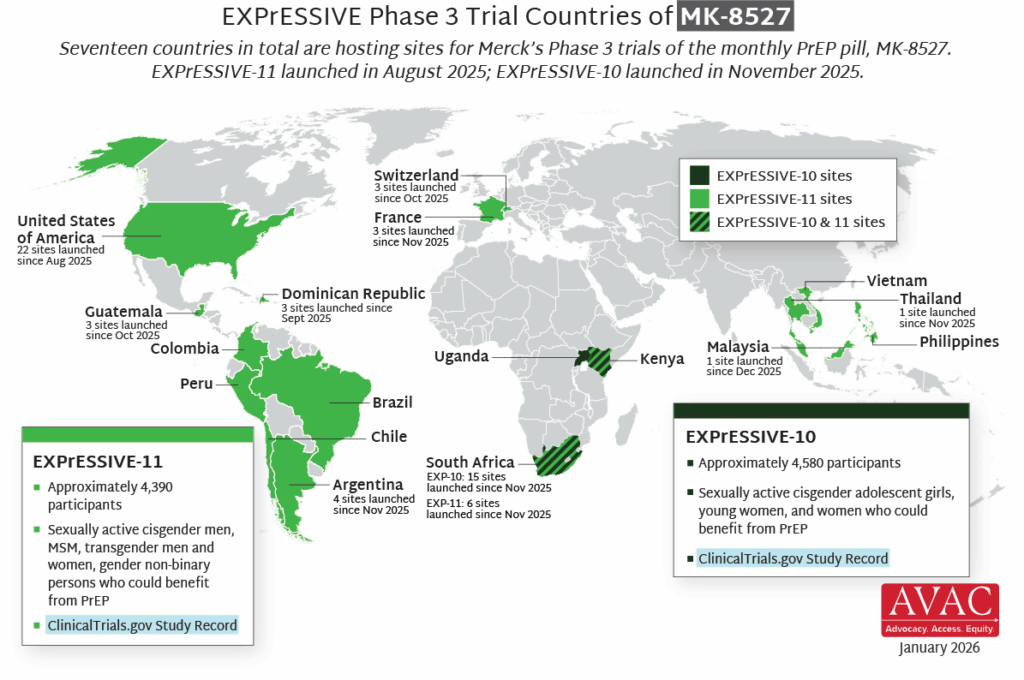
- Merck’s Phase 3 trials of the monthly oral PrEP pill MK-8527 – EXPrESSIVE-10 and EXPrESSIVE-11 – are now both underway. EXPrESSIVE-10 officially launched in November 2025 and has since begun recruitment at 19 sites in South Africa, with sites in Kenya and Uganda prepared to launch imminently. EXPrESSIVE-11, which launched last August, is now enrolling participants in 58 sites across 11 countries, with additional sites also launching soon.
- Sidaction and Aidsfonds announced funding for four projects to inform cure research, investigating the biology of viral persistence, viral control, ethics in cure clinical trials and more.
- AVAC launched its new Pipeline Tracker that is continuously updated, with comprehensive descriptions and status updates of all products in the HIV R&D pipeline.
A Deep Dive on the People’s Research Agenda
In December AVAC launched our 2025 Update to the People’s Research Agenda (PRA), a people-centered framework for tracking community priorities and progress in the pipeline of HIV prevention research and development. PRA findings include:
- 2025 saw important advances: accelerated rollout of LEN for PrEP; a once-monthly oral PrEP pill entering Phase III efficacy trials; the launch of small-scale trials of experimental vaccines; and Phase I testing of combination bNAbs.
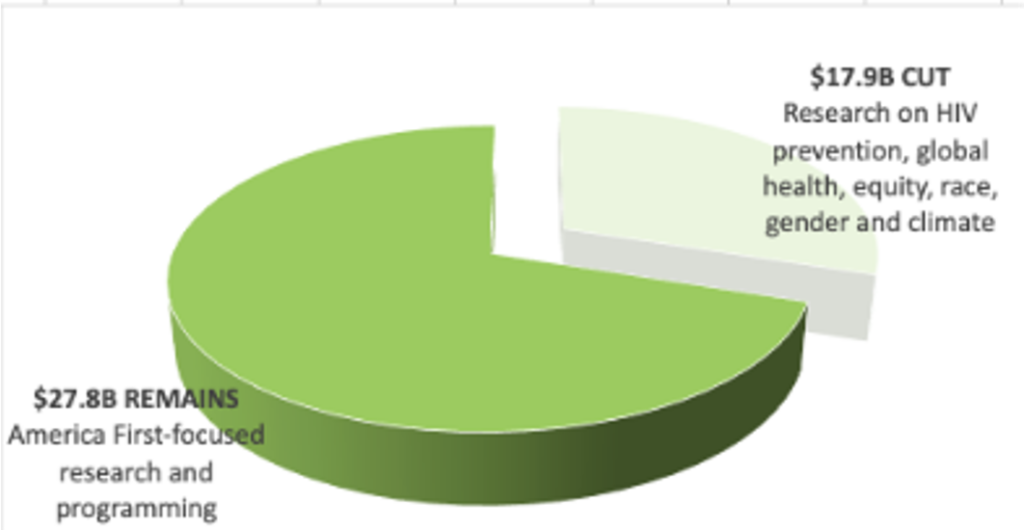
- But 2025 brought dramatic shifts for HIV R&D, with cuts and restrictions imposed on foreign aid and HIV research. Several promising trials and research programs have been terminated.
- Despite these challenges, HIV R&D must remain a priority as the global funding environment continues to evolve. The pipeline must be balanced, effective, and community-informed.
- Check out our recorded webinar here, for a showcase of the PRA with the CEO of IDSA, former director of NIAID and AVAC board member, Jeanne Marrazzo.
The Future of HIV Prevention: A People’s Research Agenda for Speed, Scale and Equity

Featured speaker Jeanne Marrazzo of the Infectious Diseases Society of America and AVAC board member explores what the People’s Research Agenda tracks and the advocacy priorities that will shape the future of HIV prevention R&D. View the recording.
“We cannot yell it from the rooftops loud enough that new infections are going to rise and undermine efforts to end AIDS as a public health threat. But this is not a time to despair. It’s a time to fight!” – Jeanne Marrazzo
Prevention Playlist
AVAC develops a wide range of resources to inform decision making and action. Check out the latest:
Join
- Conference on Retroviruses and Opportunistic Infections (CROI 2026)
- The Injectors of Tomorrow are Here Today
Use
- The People’s Research Agenda
- HIV Prevention R&D at Risk: Tracking the Impact of US Funding Cuts
- People’s Research Agenda Pipeline Tracker
- Preparing for LEN: Learning Lessons, Accelerating Access, Reducing Time to Impact
- Sign up for Global Health Watch: AVAC’s weekly newsletter to keep advocates informed, prepared and connected
- 24 Hour Marathon to Save AIDS Research
Watch/Listen
- The Future of HIV Prevention: A People’s Research Agenda for Speed, Scale and Equity
- The Quest For An HIV Cure — Will It Be Discovered in Africa?
- We Declare—Turning “The People’s Declaration” Demands into Actions and Accountability on HIV
- Do Not Check That Box — Impacts From the Assault on Transgender Communities and DEI + Strategies to Sustain and Rebuild