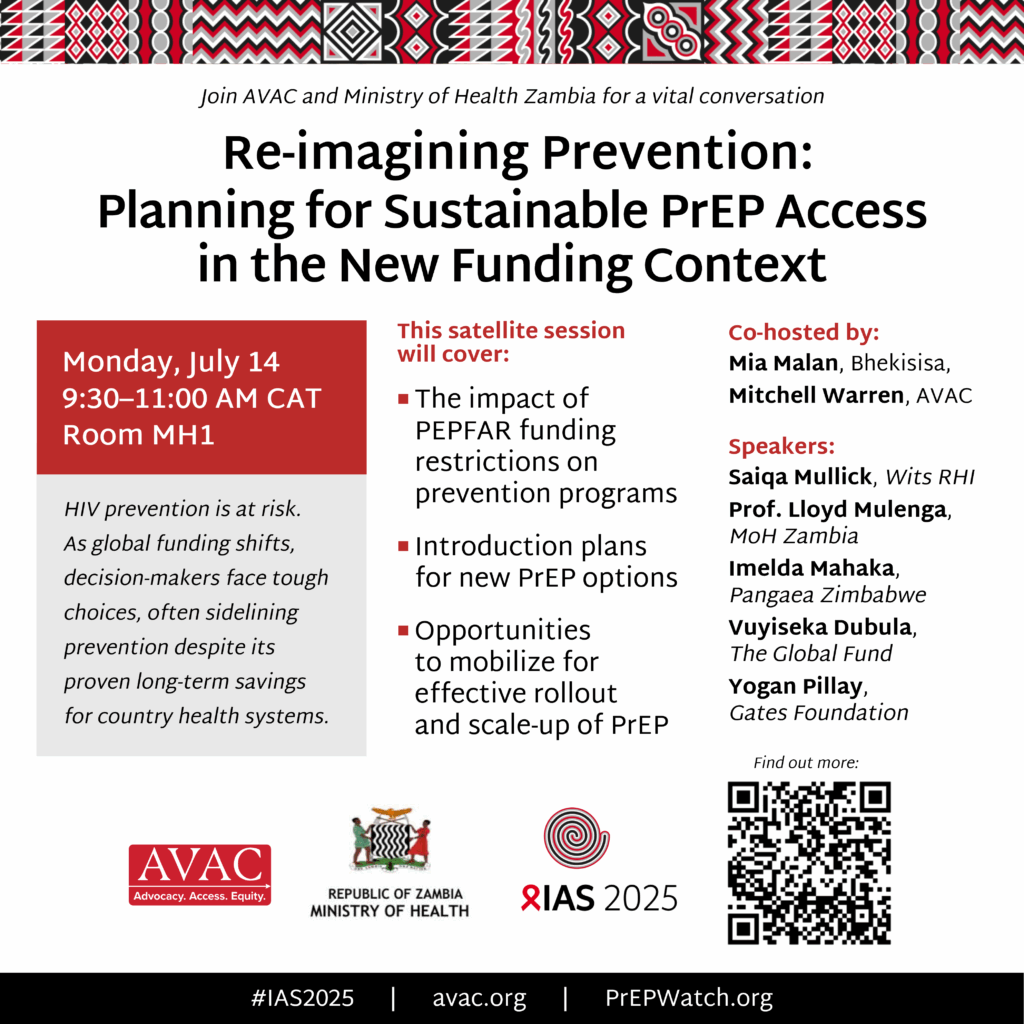
If you are attending the International AIDS Society Conference (IAS), be sure to join AVAC and the Ministry of Health, Zambia for this vital conversation.
Avac Event
If you are attending the International AIDS Society Conference (IAS), be sure to join AVAC and the Ministry of Health, Zambia for this vital conversation.

This graphic shows currently available options for HIV prevention, newly approved and recommended treatment, and those in development.
The rollout of oral PrEP demonstrates that people don’t take PrEP simply because it’s available—there needs to be a demand for it, and it needs to be accessible, acceptable and used effectively by those who need and want it. These are the lessons the field is applying to the rollout of the dapivirine vaginal ring (DVR), and injectable cabotegravir (CAB) and lenacapavir (LEN) for PrEP. To reach the UNAIDS target of 10 million PrEP users by 2025, initiations of oral PrEP alone will not be enough—and this graphic shows that the field is beginning to apply past lessons to accelerate introduction of injectable PrEP options.
For the latest on lenacapavir, visit here.
On June 11, AVAC hosted a conversation, The Scientific Journey of Lenacapavir: From basic science to clinical development to impact, to explore how US support from NIH for basic science and South Africa’s clinical research infrastructure made possible the development of lenacapavir for PrEP (LEN), a discovery in HIV prevention that went on to be named Science magazine’s 2024 Breakthrough of the Year.
As the field anticipates initial regulatory approval from the US FDA by June 19 and a WHO recommendation in July, Linda-Gail Bekker of the Desmond Tutu Health Foundation, Wes Sundquist of the University of Utah and Mitchell Warren of AVAC underscored how this moment of promise is threatened by sweeping attacks on science, research and the very systems that made the development of LEN possible.
Avac Event
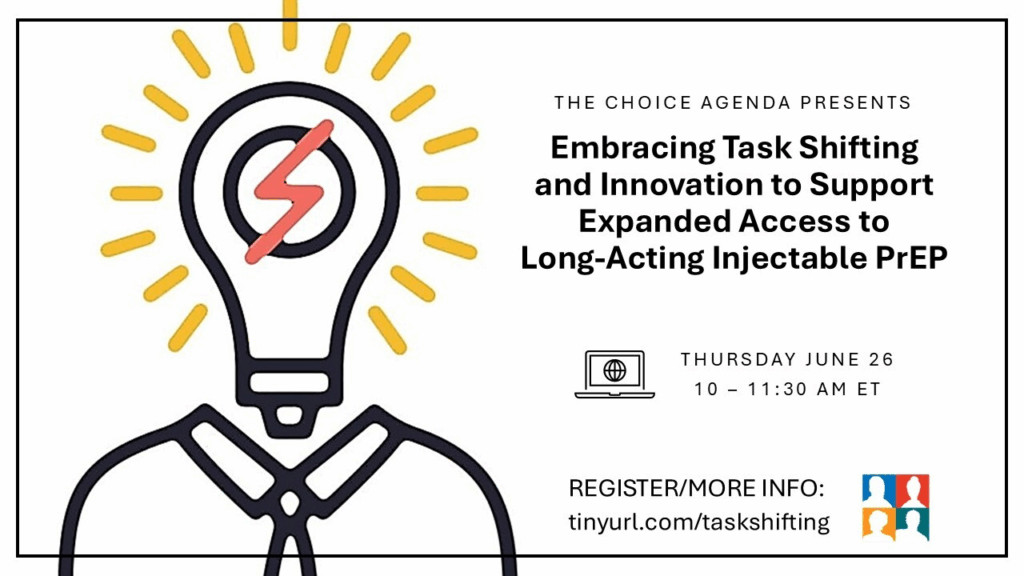
While the HIV prevention buffet will soon offer a second form of long acting injectable PrEP, ensuring access to all those who can benefit requires innovations in service delivery such as task shifting. In the United States, two Federally Qualified Health Centers (FQHCs) have implemented programming that has expanded clinic capacity, resulting in more individuals being able to choose long acting injectable PrEP. We also heard about innovative efforts to expand PrEP access in South Africa and learned what it takes to integrate task shifting for long-acting PrEP injection programs. We discussed other ways we can collectively innovate to support expanded, sustainable access to all forms of PrEP.
Speakers:
Moderator:
Materials:
Press Release
Contact: [email protected]
NEW YORK, NY, June 11, 2025—AVAC strongly condemns Secretary of Health and Human Services, Robert F. Kennedy, Jr., for removing all members of the Advisory Committee on Immunization Practices (ACIP). This committee of vaccine experts—with decades of experience in vaccine development, delivery and safety—is responsible for developing the country’s vaccine policies and recommendations for the Centers for Disease Control and Prevention (CDC). At a time when science is under political attack and vital programs are being defunded, AVAC stands with researchers, advocates, and communities calling for Congress to defend public health and unbiased science, which is essential to safeguarding the health of all Americans. The Secretary’s actions attack the integrity of ACIP membership and is a direct threat to public trust in our health systems and in the essential role of vaccines in disease prevention.
“Vaccines remain among the most powerful public health tools ever developed,” said Mitchell Warren, executive director of AVAC. “Vaccines have transformed the global response to infectious diseases, from smallpox to measles to COVID-19, and they are central to the vision of ending the HIV epidemic. At a moment when the US should be investing more in vaccine science, access, and public confidence, it is investing less and simultaneously undermining vaccines generally. Secretary Kennedy’s short-sighted and unceremonious removal of all ACIP members is an alarming escalation in this administration’s campaign to dismantle evidence-based health policy, science and research.”
ACIP’s long-standing commitment to base vaccine recommendations purely on the evidence represents the highest standards of ethical guidance to protect human health. The Secretary’s decision undermines not just US vaccine strategy, but global confidence in immunization programs and guidance that have long relied on US leadership.
The destruction of ACIP adds to the five-month litany of assaults on vaccines and the systems that support them. From proposed cuts to the Fiscal Year 2026 budget, to the defunding for global vaccine access programs like Gavi and domestic immunization initiatives at the US CDC, to the undermining of the essential role of measles vaccines, the closure of the leading NIH-funded HIV vaccine consortia (CHAVD), to the dismantling of USAID’s HIV vaccine R&D programs and the recommendation to remove COVID-19 vaccines from the US immunization schedule for children and pregnant women, this administration has worked to subvert the importance and impact of life-saving vaccines and erode public trust for vaccine science.
Vaccines work. Human papillomavirus (HPV) is responsible for 99% of cervical cancers. The HPV vaccine prevents over 90% of cancers caused by HPV, including anal, cervical, penile, throat, vaginal, and vulvar globally, and if more widely available, could prevent hundreds of thousands of annual deaths, according to the WHO. Similarly, hepatitis B (HBV) accounts for 1.1 million deaths globally, and yet the HBV vaccine prevents as many deaths every year, according to WHO. Furthermore, without a vaccine review panel, rollout of anticipated vaccines that protect against gonorrhea, could make access difficult for many Americans when drug resistant gonorrhea is on the rise globally.
“We are witnessing other countries eliminate cervical cancer through robust HPV vaccination and screening programs while the US risks reversing decades of progress,” says Alison Footman, AVAC’s senior program manager of STIs. “The ACIP plays a critical role in ensuring vaccines, including those that prevent STIs like HPV and hepatitis B, are accessible, and recommendations are guided by expert opinions. Now more than ever, we must protect the integrity of public health systems that save lives and prevent diseases.”
As public confidence in vaccines erodes, the value of vaccine science is paramount, representing one of the single greatest advances in the history of medical science, eradicating once life-threatening infections and mitigating the risk of illness from numerous diseases. In the field of HIV, the search for an effective vaccine is advancing thanks to decades of investment in basic science, clinical research, and global partnerships. This progress must be protected and accelerated. A vaccine would provide a durable, scalable form of HIV prevention that does not rely on frequent adherence or health system access, and it would be especially transformative for communities most marginalized by current systems.
AVAC calls on Congress, scientists, and civil society to speak out and stand up for science, for vaccines, and for the future of global and public health.
###
AVAC is an international non-profit organization that provides an independent voice and leverages global partnerships to accelerate ethical development and equitable delivery of effective HIV prevention options, as part of a comprehensive and integrated pathway to global health equity. Follow AVAC on Bluesky and Instagram. Find more at www.avac.org and www.prepwatch.org.
Avac Event
This High-Level Dialogue organized by the Global HIV Prevention Coalition and co-hosted by UNAIDS in collaboration with UNFPA, WHO and UNDP, the Federal Republic of Brazil and Kingdom of the Netherlands aims to galvanize political leadership, financing, and coordinated action to drive a transformational HIV prevention push. The meeting will serve as a platform for Ministers of Health, global health partners, pharmaceutical companies, and civil society to explore opportunities to expand access to new long-acting prevention technologies as a powerful addition to existing effective options.
Join Mitchell Warren as he chairs the discussion alongside UNAIDS leadership, representatives of UN partners and global health stakeholders.
The US presidential administration is actively working to dismantle HIV research and demolish the architecture of global health. The entire HIV response — from basic research and clinical development to policy, programs, and global access to life-saving treatment and prevention — is now under attack, and the world runs the risk of reversing the strides made to end HIV.
AVAC has put together this report, highlighting the impact of US cuts on the pipeline of HIV prevention research and development. AVAC will continue to track these cuts and their impact, to amplify the damage they will cause, and to fight for their reversal.
Avac Event