This timeline shows the potential time points when the next-generation of HIV prevention options might find their way into new programs.
Years Ahead in HIV Prevention Research: Time to Market
PxWire Volume 15, Issue 3
With the recent FDA approval of injectable lenacapavir (LEN) for PrEP and the drastic withdrawal of US investment in HIV prevention, the field must reimagine and recommit to getting PrEP rollout right this time AND to sustaining the HIV research pipeline. Research on HIV has brought numerous advances to global health, but controlling, and ultimately ending, the epidemic depends on continued investment in innovation.
This issue of PxWire looks at the scale of shuttered prevention programs for key populations (KPs), the potential market for injectable LEN, and the devastating cuts to research for an HIV vaccine.
Read below or download the PDF version of this issue.
Progress in PrEP Uptake
The PEPFAR stop work orders issued by the US government in January 2025 have devastated the HIV response worldwide, including funding for primary prevention. Sustaining the HIV response and bending the curve of incidence depends on identifying new sources of funding to maintain HIV prevention programs for KPs.
- Guidance issued in February 2025 indicated that PrEP services funded by the US government are permitted only for pregnant and lactating people—meaning that KPs, such as LGBTQ+ individuals, sex workers, and people who use drugs, have lost access to PrEP, unless they are currently pregnant or lactating.
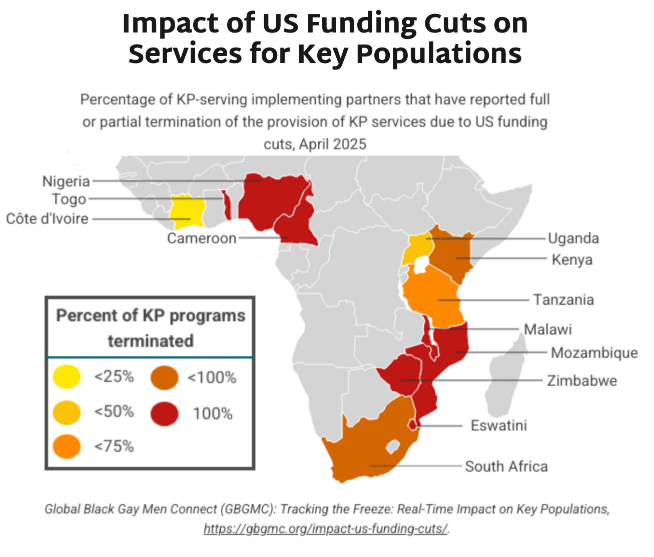
- The graphic below shows key findings on the percentage of KP programs terminated by country. Most of the priority African countries for the HIV response report national KP programs are fully or partially terminated.
- HIV incidence rates amongst KPs are higher than in other populations. Excluding this group from PrEP programming endangers whole communities threatened by HIV, disables the HIV response, and jeopardizes gains made against the epidemic.
- Research undertaken by Global Black Gay Men Connect (GBGMC) examines the impact of US government funding cuts on KP programs in Africa.
- Additional research done by AVAC, in the report Impact of PEPFAR Stop Work Orders, shows that KPs are the group most impacted by US government funding reductions to HIV prevention services worldwide. In some cases, such as Panama, national governments are stepping in to fill the gap.
PrEParing for New Products
Stakeholders—including Global Fund, PEPFAR, WHO, UNAIDS, Unitaid, Ministries of Health, advocates and implementing partners—have critical work to do now to ensure doses of LEN hit the ground as quickly as possible. Check out Gears of Lenacapavir for PrEP Rollout and Getting PrEP Rollout Right This Time to get the details.
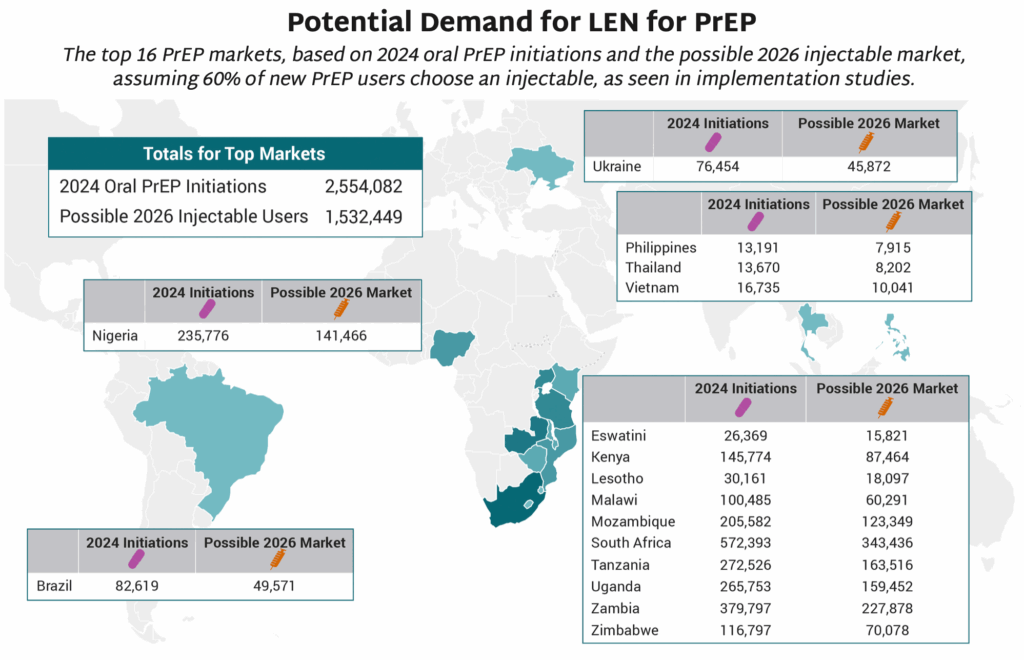
- The map shows 16 countries in Africa, Asia and Latin America that have the largest PrEP markets.
- If total PrEP initiations continue to increase by 20% every year, which is the trend in recent years, and injectables represent 60% of initiations, as seen in implementation studies, 2026 numbers of injectable initiations could be as shown in the map.
- The exact price and volumes of LEN per country is not yet known.
- Of those injectable initiations, LEN is expected to be the majority, given the proposed manufacturing projections from Gilead and the stated ambition of the Global Fund to reach two million people with LEN within the first three years of introduction. Additional volumes of injectable cabotegravir would make up the rest.
- PEPFAR, which in December committed to collaborate with Global Fund, has not yet publicly stated how LEN will fit into their more limited approach to PrEP, which has been restricted to pregnant and breastfeeding women.
The Latest R&D in the Prevention Pipeline
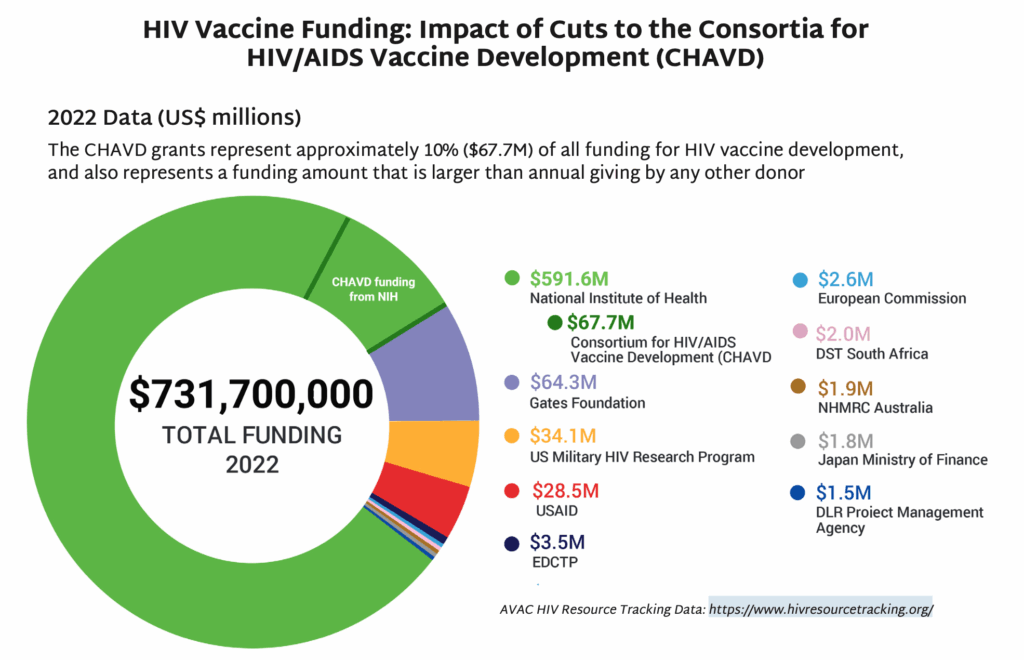
- In May, NIH’s National Institute of Allergy and Infectious Diseases (NIAID) announced that funding for the Consortia for HIV/AIDS Vaccine Development (CHAVD) would end after the current grant cycle in June 2026. With only one more year of funding before the grants end, current plans for research, clinical trials and progress toward a vaccine are all at risk.
- First launched in 2005, the CHAVDs led ground-breaking research to develop an HIV vaccine.
- CHAVD grants currently fund two institutions as consortia leaders—The Scripps Research Institute and Duke University.
- The quest for an HIV vaccine is gaining momentum with field-changing contributions from the CHAVDs. The institutions are currently researching vaccine designs that rely on the immune system’s broadly neutralizing antibodies (bNAbs) to protect against HIV.
- The annual funding for the consortia—approximately $67M—represents a significant chunk of the NIH’s funding for HIV vaccine development, and also approximately 10% of all funding for HIV vaccine research globally each year.
- NIH’s yearly grant total for the CHAVD is larger than any other individual donor’s annual giving for HIV vaccine research. The next closest donor is The Gates Foundation, which donates approximately $64M a year to this research.
- In 20 years of research, CHAVD discoveries have resulted in new technology to combat HIV, influenza, Zika, COVID, and other novel coronaviruses. The loss of the CHAVD will have a devastating impact on the HIV response and scientific discovery.
Prevention Playlist
AVAC develops a wide range of resources to inform decision making and action. Check out the latest:
Join
- CHANGE: In response to the unfolding crisis, more than 1,500 people from civil society organizations around the world have launched CHANGE—Community Health & HIV Advocate Navigating Global Emergencies—a coalition formed to support urgent action: [email protected]
- Subscribe to Global Health Watch: AVAC’s weekly newsletter dedicated to breaking down critical developments in US policies and their impact on global health, at avac.org/global-health-watch
- Fight For Our Lives” Emergency Townhall: Impact of the Trump Administration Foreign Aid Freeze on KP & LGBTQ Communities, Ongoing convening, Register here
Use
- Advocacy Resources for Injectable LEN, AVAC
- HIV Prevention R&D at Risk, AVAC
- Impact of PEPFAR Stop Work Orders, AVAC
- Research Matters Advocacy Toolkit, AVAC, TAG, HIVMA
- Advocates’ Guide: Understanding the President’s Proposed Fiscal Year 2026 (FY26) Budget and Its Implications for Science, Research and Global Health, AVAC
Watch/Listen
- FDA Approves Injectable LEN for PrEP
- Fight for Firewalls: HIV and Health Data Privacy in the Snowballing Surveillance State, Webinar
- Embracing Task Shifting and Innovation to Support Expanded Access to Long-Acting Injectable PrEP, Webinar
- The Scientific Journey of Lenacapavir: From basic science to clinical development to impact, Webinar
- Critical Advocacy: How Civil Society is defending the HIV Response and Global Health, Podcast
- HIV Prevention at a Crossroads: Why we still need an HIV vaccine, Webinar
- A New Era of HIV Prevention: Accelerating access to long-acting prevention options through sustainable prevention systems and financing, Webinar
Read
- FDA Approves Injectable Lenacapavir for PrEP, AVAC
- Trump Aid Cuts Deal a Blow to HIV Prevention in Africa, Reuters
- Will long-lasting HIV preventive be a game changer—or a missed opportunity?, Science
- Regulators Approve a Twice-Yearly Shot to Prevent HIV Infection, New York Times
- FDA Approves Twice-Yearly Lenacapavir for HIV Prevention, POZ
- FDA approves twice-yearly shot for HIV prevention, Helio
- BREAKING: FDA approves breakthrough drug that reduces risk of contracting HIV by 96 percent, The Advocate
- Getting PrEP Rollout Right This Time: Lessons from the field, AVAC
- Why STI Funding Matters, AVAC
- AVAC Condemns Removal of the Advisory Committee on Immunization Practices, AVAC
- AVAC Denounces White House Effort to Codify DOGE Cuts to Health, Research and Foreign Assistance, AVAC
- Protect Federal Funding for HIV, TB, and STI Research and Prevention at the National Institutes of Health, AVAC
- The People’s Research Agenda, AVAC
- Worldwide Prevention, Shared Protection: Why STI Funding Matters, AVAC
The Cruel Irony of Prevention
It’s been 22 weeks since the US president issued the executive orders that began the chaotic dismantling of USAID. See recent, remarkable, and much-needed New York Times coverage on the destruction of USAID. And check out this new episode of the This American Life podcast on the aftermath of shutting down USAID.
We at AVAC took a public stand against this attack on global health and development in our court case, AVAC v. United States Department of State, filed by Public Citizen on February 10. We took this step because we understood the devastation that would come from such drastic and sudden divestment in the HIV response and global health. We also believe it was illegal and unethical.
In March, US District Court Judge Amir Ali ruled the foreign aid freeze was, indeed, unlawful, and that the administration had “usurped” the authority of Congress. Since that time, some 400 grants have been restored and the government is slowly complying with the Judge’s order to pay all invoices for work conducted through February 13. It’s a mere fraction of USAID’s former self, but each of these restored programs and payments represent an attempt to make America and the world stronger, safer and more prosperous. Just as important, our court case, along with a related one brought by the Global Health Council and colleagues, has forced into the public record the administration’s cynical maneuvers to dismantle global health; information that empowers advocates to fight and, we hope, finally spurs Congress to do its job.
“We cannot cede ground gained against HIV and other global health threats out of fear or paralysis in the face of these reckless actions. It is imperative to hold this administration responsible. And it’s imperative to invest in global health and sustain the gains in HIV. Global health advocates know this better than anyone, and we are fighting back,” said AVAC Executive Director, Mitchell Warren.

Watch AVAC’s Executive Director, Mitchell Warren’s video explaining how this court case is one of many critical steps to safeguard global health and the HIV response.
Nowhere is that fight to safeguard and advance HIV prevention more important than work to rollout injectable lenacapavir (LEN) for PrEP, just approved by the US Food and Drug Administration. LEN approval signals what could be a turning point in the epidemic – but only if the field invests in bold, strategic action.
“The science is remarkable, and the FDA approval is in. But it’s what happens next that makes the science count. It’s whether or not the technology is made available and becomes truly accessible that will bring impact. Will the world get it right this time? Because for 15 years we’ve squandered the potential of PrEP. That hope depends on fierce and effective advocacy,” said Warren.
HIV prevention advocates around the world are demanding and preparing for injectable LEN to reach the communities that need it most with speed, scale and equity. Watch this video for more on status of LEN rollout and advocacy priorities.
“Scientific progress has been made over the years, and we celebrate them, but without truly having a prevention story to tell…yet. Now it’s time for that story to be told,” said APHA Executive Director Yvette Raphael.
Read this report from Friends of the Global Fight Against AIDS, Tuberculosis and Malaria, Principles of a responsible transition of American leadership to end AIDS: Strategic transition or pandemic resurgence?, on how PEPFAR and US leadership can play an instrumental role in reaching epidemic control.
“There’s much work advocates are doing, and much work ahead. Some donors have come forward, but it’s only a start. We also need to advocate with regulatory authorities at the country level, not to mention pushing for an affordable price, and a big enough supply from Gilead now to support demand creation at scale in order to build a sustainable market, which in turn will support generics and further reductions in price. Advocates are now and will continue to be fighting at the country level and the global level to move all this forward,” said HEPS Uganda Executive Director Kenneth Mwehonge and co-chair of the Civil Society Caucus of the Coalition to Accelerate Access to Long-Acting PrEP.
Check out the Caucus statement on priorities for LEN rollout, and these resources to support our collective advocacy:
Recent news headlines, including the just published New Yorker magazine article by Science reporter Jon Cohen, capture the power of this moment to reshape the HIV response and finally control the epidemic. As advocates, we know the stakes very well.

“What all of us working together have tried to do for years and years and years, is to overcome the barriers from stigma to disinformation, that we’ve seen with oral PrEP, and with treatment 20 years ago. It will take Gilead, working with all of us advocates, to collectively ensure that this drug is accessible to all, so that the success depends not on the politics but on the product. We have to hold everyone, including ourselves, to account for keeping on task and for making sure that we do not squander this opportunity. So, let’s do this together and lay the groundwork for all future innovations to go faster, with speed, with scale and with equity for all,” Warren said.
The field is at a major inflection point, punctuated by both political challenges and scientific opportunity – and we can’t let the former overtake the latter.
Advocates’ Guide to Lenacapavir
This wide-ranging slide deck gives a complete overview of lenacapavir — showing the overall prevention product pipeline, describes lenacapavir, compares it to other options, discusses the trials testing the product, next steps, and links to advocacy resources.
The HIV Prevention Pipeline
This graphic shows currently available options for HIV prevention, newly approved and recommended treatment, and those in development.
Where We Are Now with LEN for PrEP
The chaos in foreign assistance programs (including discontinuation of major PrEP programs), cuts in staffing and new demands on donor commitments will make decisions on the procurement of LEN for PrEP more complex and uncertain.
In December 2024, the Global Fund and PEPFAR announced a plan to reach 2 million people with LEN for PrEP over three years. Exactly how funding to support this unprecedented introduction program will move forward, in the absence of significant US investment, is far from certain. The other stakeholders, including Global Fund, Gilead, CIFF and the Gates Foundation expressed commitments to the deal, but major questions remain.
Moving a Product to the Real World
The rollout of oral PrEP demonstrates that people don’t take PrEP simply because it’s available—there needs to be a demand for it, and it needs to be accessible, acceptable and used effectively by those who need and want it. These are the lessons the field is applying to the rollout of the dapivirine vaginal ring (DVR), and injectable cabotegravir (CAB) and lenacapavir (LEN) for PrEP. To reach the UNAIDS target of 10 million PrEP users by 2025, initiations of oral PrEP alone will not be enough—and this graphic shows that the field is beginning to apply past lessons to accelerate introduction of injectable PrEP options.
For the latest on lenacapavir, visit here.
An Overview of Lenacapavir for PrEP Trials
Lenacapavir Regulatory Approval
Regulatory approvals, pending decisions, and appeals as of June 2025, including US Food and Drug Administration approval. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Press Release
FDA Approves Injectable Lenacapavir for PrEP
A Historic Milestone Must Now Be Matched by Urgent Action
Contact: [email protected]
New York, NY, June 18, 2025 — AVAC welcomes the U.S. Food and Drug Administration (FDA) approval of injectable lenacapavir (LEN) for the prevention of HIV as pre-exposure prophylaxis (PrEP). LEN, developed by Gilead Sciences, is a twice-yearly injectable PrEP option that showed nearly complete protection against HIV in the landmark PURPOSE 1 and 2 trials. Science Magazine named LEN the “Breakthrough of the Year” in 2024, a recognition that reflects its enormous potential. But that promise will only be realized if it is rolled out with speed, scale, and equity.
“The approval of LEN is a much-needed boost for HIV prevention, given the strength of the science and the simultaneous disruption in HIV programs globally,” said Mitchell Warren, executive director of AVAC. “But US FDA approval is just one in a series of steps needed to ensure that injectable LEN can help reduce the 1.3 million new HIV infections that occur each year. Scientific progress only matters if innovation actually reaches people. LEN for PrEP is poised to re-shape the HIV response, but only if today’s approval is accompanied by bold, strategic, effective and equitable rollout that reaches the populations that need access. Otherwise, the world risks squandering this PrEP opportunity, as it has with other PrEP options too often over the past 12 years.”
In December, PEPFAR and the Global Fund announced a coordinated ambition to reach two million people within three years of product launch. This commitment signals an unprecedented opportunity to make PrEP access a reality. But translating this ambition into impact, especially now amid the current political environment, is not without considerable challenges.
“Political will, programmatic implementation, and sustainable funding are needed to truly accelerate equitable and impactful introduction of LEN worldwide,” said Wawira Nyagah, AVAC’s director of product introduction & access. “We have over a decade of hard-won lessons on what it takes to rollout PrEP effectively, and the field cannot afford the delays we have seen with the past launches of daily oral PrEP, the monthly dapivirine vaginal ring (DVR), and every-two-month injectable cabotegravir (CAB). Lives depend on speed, scale and equity.”
The World Health Organization (WHO) is expected to release updated PrEP guidelines for LEN in July, and regulatory agencies in Brazil, Europe and South Africa are simultaneously reviewing the product. But the current political context, including a shuttered USAID and further disruptions across global health, demands an urgent and courageous response. In January, the US Administration issued a stop-work on all USAID-funded grants, nearly paralyzing HIV treatment and prevention by PEPFAR, the primary funder of programs in HIV-burdened countries (and administered by USAID). In February, PrEP was broadly excluded from a waiver that allowed HIV treatment to continue and allowed PrEP only for pregnant and breastfeeding women. These policies could not only undercut LEN’s promise but roll back years of progress in HIV prevention.
It will take new, re-vitalized and committed partnerships to work together to sustain past progress and advance HIV prevention to deliver on the UN targets for epidemic control. AVAC’s The Gears of Lenacapavir for PrEP Rollout outlines the steps needed from national governments, funders, researchers, drug-makers including generic manufacturers, and civil society to ensure LEN reaches those who need it most. In the near term, these stakeholders each have vital work to do to complement the initial announcement from the Global Fund and The Children’s Investment Fund Foundation (CIFF) in their pledged collaboration to significantly expand access to LEN for PrEP.
“No one donor, national government or manufacturer can realize this ambition alone,” said Warren. “All stakeholders—including Gilead, PEPFAR, and the Gates Foundation—must act decisively to seize this opportunity, ensuring that all populations—regardless of geography, income, or identity—benefit from this innovative prevention option.”
Meeting this moment requires funders, Ministries of Health, implementers and civil society partners to collaboratively design a comprehensive introduction strategy that breaks the sequential nature of traditional approaches to scaling up interventions. Instead, to speed up introduction, stakeholders must move toward a parallel approach where research, implementation science, and programs at scale are designed, funded and implemented simultaneously. This introduction strategy should entail:
- Other funders and national governments to join the Global Fund and CIFF and commit to procure at least enough LEN from Gilead for two million person years of protection beginning this year through 2027.
- Gilead to set a cost-effective price that compares to generic daily oral TDF/FTC. Achieving this will require a low launch price from Gilead, significant volume procurement from donors, and the entry of multiple generic manufacturers into a competitive, multi-million-user market. While this low price is not expected at launch, stakeholders must act now to reach this price point as quickly as possible by building volume with supplies from Gilead at no more than $100 per person per year and to support multiple generic manufacturers to enable production at larger scale and lower prices as quickly as possible.
- A mobilized civil society in high-burden countries pushing national governments to expedite regulatory approvals, integrate LEN into HIV and national health programs with domestic resources, and develop national guidelines without delay.
- Civil society also demanding transparent pricing and a clear, accelerated pathway to sustainable PrEP programs—so that by the time generic LEN becomes available around 2028, the market is primed for rapid scale-up, with multiple producers driving down prices through competition.
- Gilead working with their generic license holders to accelerate production and expand generic availability in middle-income countries.
- The US Administration, via the State Department, releasing all appropriated funds, negotiate best prices at scale and provide LEN to all who need it. These actions are essential to achieve a strategic transition and sustainability against the global HIV epidemic.
“This is the moment to build on the momentum of science, which has brought the field to this day, when LEN for PrEP is speeding through regulatory review faster than any prevention product to date,” said Nyagah. “Translating this success into real impact on the epidemic, led by communities around the world, must be a top priority among all stakeholders.”
###
About AVAC: Founded in 1995, AVAC is an international non-profit organization that provides an independent voice and leverages global partnerships to accelerate ethical development and equitable delivery of effective HIV prevention options, as part of a comprehensive and integrated pathway to global health equity. Follow AVAC on Bluesky and Instagram. Find more at www.avac.org and www.prepwatch.org.