New York City, September 12, 2024 – AVAC welcomes the groundbreaking results of the PURPOSE 2 HIV prevention study of twice-yearly injectable lenacapavir for PrEP among 3,200 cisgender men, transgender men, transgender women, and nonbinary individuals who have sex with partners assigned male at birth. Among more than 2,000 people in the trial who received lenacapavir, there were only two HIV infections.
Preliminary safety and efficacy results were reported today by Gilead Sciences, the drug’s developer. An independent data and safety monitoring board (DSMB), at a scheduled review of the trial data, found the regimen to be safe and highly effective, with a 96% lower HIV rate compared with the expected background incidence of HIV infection and 89% lower compared with oral TDF/FTC. These results follow the results of PURPOSE 1, released earlier this year, that showed 100% efficacy of lenavcapavir among cisgender women in South Africa and Uganda.
“This is the second impressive result for this new HIV prevention option, opening up more possibilities for choice for even more people to find an option that is right for them,” said Mitchell Warren, AVAC’s executive director. “Beyond expanded choice, a twice-yearly injection has the potential to transform the way we deliver HIV prevention to people who need and want it most – from an easier to follow regimen for individuals to a decreased burden on healthcare systems that are stretched to the limit. But these data only matter if the field moves with speed, scale and equity.”
“Having results from a trial population that includes trans men and women, nonbinary people and gay men is an important milestone for community inclusion in HIV prevention studies,” said Kenyon Farrow, AVAC communications director and PrEP user since 2015. “I am excited that people who want to use PrEP or who fear stigma or discrimination, may soon have the possibility of another option that could be much easier to use and provide more discretion. It is imperative that we accelerate planning for rollout of lenacapavir. We know that even with the most ambitious timeline, it will take time for lenacapavir to be rolled out to all who need and want to use it.”
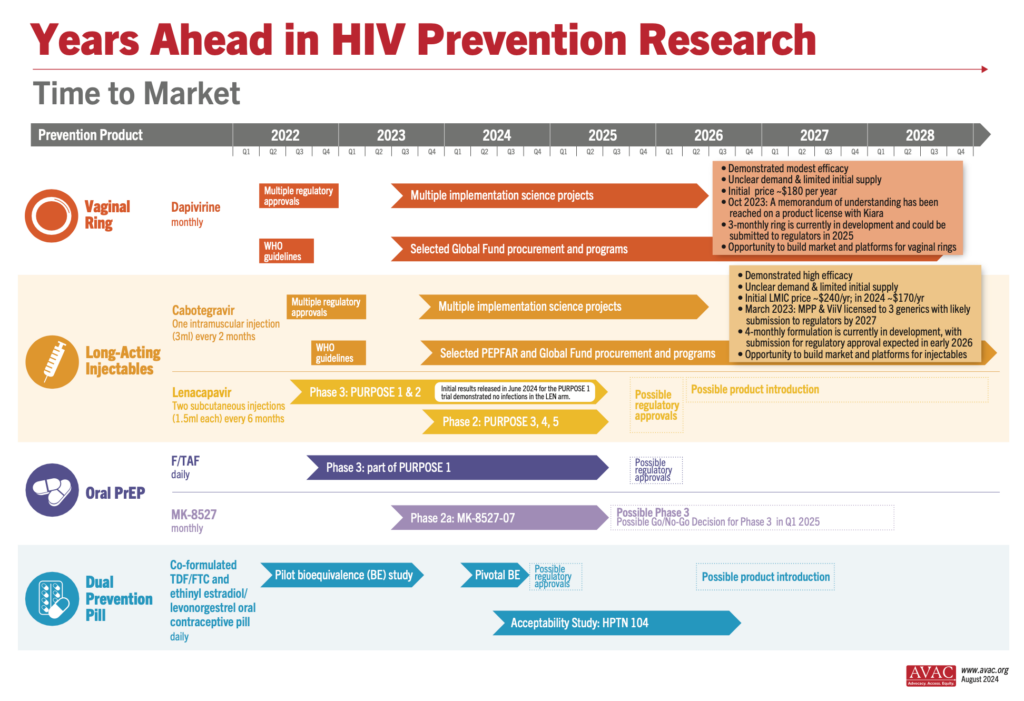
The study evaluated the safety and efficacy of twice-yearly injectable lenacapavir for PrEP compared to once-daily oral emtricitabine/tenofovir and background HIV incidence. All trial participants will now be offered lenacapavir. Additional studies in critical populations, including PURPOSE 3 among cisgender women in the US and PURPOSE 4 among people who inject drugs, are also underway. It will be imperative to understand how today’s results influence these trials. A schematic of the suite of studies is here.
“We applaud Gilead’s commitment to Good Participatory Practice in this and the other PURPOSE studies, especially through the inclusion of multiple populations in the PURPOSE studies and a commitment to including community voices in trial design and in access plans” said Stacey Hannah, AVAC director of research engagement. “Access and implementation plans must be shaped and informed by continuous, robust participatory engagement. AVAC and our partners look forward to continuing engagement with Gilead and other key stakeholders in this process.”
Importance of Access and Equity
Gilead said it is committed to making lenacapavir available for prevention in countries where it is needed most and to granting direct voluntary licenses for longer-term availability. Today’s results make it clear that Gilead, along with regulatory and normative agencies, funders and civil society, must work on an accelerated timeline to ensure broad and timely access to individuals and communities everywhere.
“In an updated access statement today, Gilead committed to beginning global regulatory filings by the end of 2024 and to facilitating faster access to target populations and countries,” Warren said. “This raises the stakes to accelerate speed, scale and equitable access. Gilead needs to urgently grant these licenses even before regulatory approval and name its prices, so that funders can prepare to accelerate product introduction. And WHO must urgently initiate its guideline review process so that lenacapavir, if approved by regulatory agencies, can be immediately added into the PrEP method mix. There is no time to waste if we are to translate these exciting clinical trial results into actual public health impact.”
“We now know that lenacapivir for PrEP is safe and highly effective among a range of populations,” Farrow added. “Even as we await regulatory submission and review, there is urgent work to be done now by communities, policy makers, funders and program implementers to design and build HIV prevention programs and prepare health systems to deliver the growing array of biomedical PrEP options, including the addition of twice-yearly injectable lenacapavir. The full range of PrEP products—including oral PrEP—must be made feasible choices for all people who need and want HIV prevention options.”
Lessons learned from rollout of daily oral PrEP, and more recently the dapivirine vaginal ring and injectable cabotegravir, can help speed regulatory approval and guideline development in key countries, design of effective programs, and community understanding of and acceptance of lenacapavir for PrEP.
“For many years, AVAC and a coalition of international partners have been planning for successful, accelerated introduction of PrEP at scale and with equity. There can be no excuses, no delays, and no repeats of the failures of oral PrEP rollout. We must move with speed, scale, and equity to ensure lenacapavir has the impact we need to prevent new HIV infections,” said Warren.
###
About AVAC
AVAC is an international non-profit organization that provides an independent voice and leverages global partnerships to accelerate ethical development and equitable delivery of effective HIV prevention options, as part of a comprehensive and integrated pathway to global health equity. Follow AVAC on Twitter @HIVpxresearch; find more at www.avac.org and www.prepwatch.org.