This graphic shows currently available options for HIV prevention, newly approved and recommended treatment, and those in development.
The HIV Prevention Pipeline
Avac Event
Innovative HIV Prevention and Treatment Keynote and Panel Discussion
An in-depth discussion on lenacapavir, this session will examine its potential to transform HIV prevention on a global scale, looking at the latest clinical evidence, regulatory pathways, and the access barriers that may shape its rollout, especially in low-resource settings. The program will feature a special keynote address followed by a panel discussion with leading experts in HIV prevention and global health, exploring how innovation, policy, and equity intersect in the next frontier of HIV response.
Keynote Session
- Moupali Das, MD, MPH, Gilead Sciences
- Keynote Moderator: Dr. Charles B. Holmes, Director, Georgetown University Center for Innovation in Global Health; Distinguished Scholar and Program Director·O’Neill Institute for National and Global Health Law at Georgetown University Law Center
Panel Discussion
- Moderator: Martha Sichone Cameron, PhD Candidate, Global Infectious Disease, Georgetown University
- Micheal Ighodaro, Executive Director, Global Black Gay Men Connect (GBGMC)
- Rupa Patel, MD, MPH, Clinical Researcher, Whitman Walker Institute
- Mitchell Warren, Executive Director, AVAC

PxWire Volume 15, Issue 4
This issue provides a range of maps to help orient the field on critical HIV prevention activities: the status of delivering injectable cabotegravir (CAB for PrEP); funders and countries on track for early introduction of injectable lenacapavir (LEN for PrEP); and where new Phase 3 efficacy trials testing MK-8527 as a monthly pill for PrEP are taking place.
Read below or download a PDF version of this issue.
Progress in PrEP Uptake
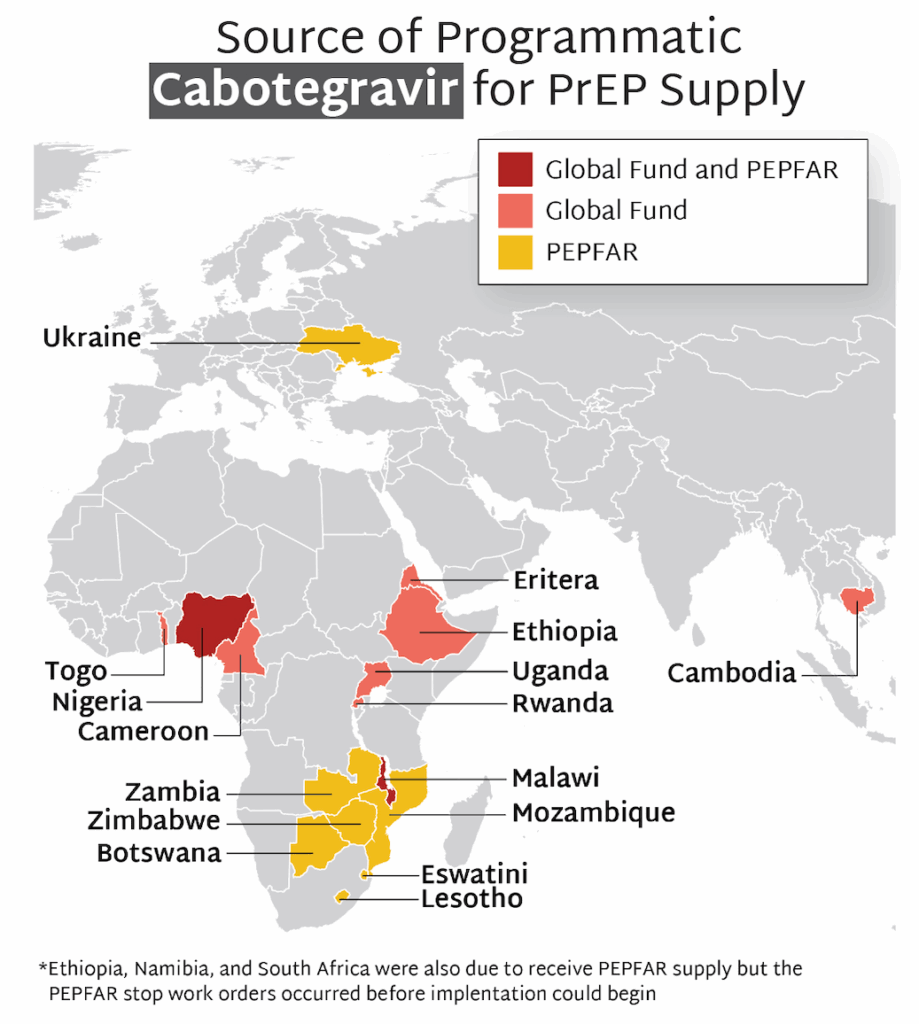
- The first supplies of CAB for programmatic use (as opposed to use in implementation studies) began to arrive in countries in 2024.
- Currently, 16 countries are rolling out CAB for programmatic use, with the majority of supply provided by PEPFAR, and some additional quantities procured by the Global Fund.
- Stop work orders for PEPFAR programming, issued in January 2025 by the new US administration, led to the cancellation of planned programs to offer CAB before they could start in Namibia and South Africa. Ethiopia’s CAB program was able to move forward with supplies supported by Global Fund.
- Stop work orders disrupted ongoing CAB programs in other PEPFAR supported countries, too. They were able to resume, though in some cases at a reduced level. Global Fund supported programs were unaffected by stop work orders and are ongoing.
- The future of PEPFAR and Global Fund supported CAB programs remains uncertain in the context of multiple forms of injectable PrEP entering the market. But lessons and insights being gathered now on delivery of injectable PrEP via these programs can be leveraged to help countries prepare for the introduction of LEN.
- In the past quarter, CAB for PrEP received additional regulatory approval in Argentina, Chile, Mexico, and Rwanda.
PrEParing for New Products
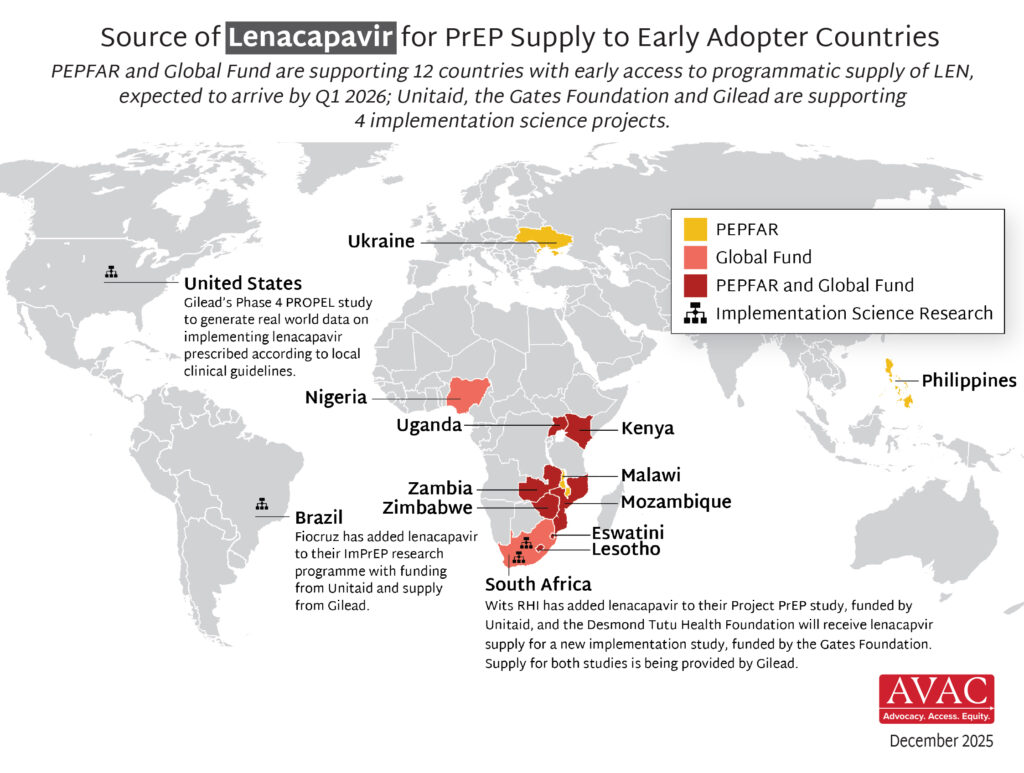
- The Global Fund, with support from CIFF, and PEPFAR have jointly committed to reaching up to two million people with LEN for PrEP over three years.
- Supply of LEN is due to begin arriving in countries in late 2025 with service delivery planned to start in early 2026.
- Ministries of Health, implementing partners and communities are now preparing for LEN introduction, and CIFF, Unitaid and PEPFAR are funding technical support efforts. PrEPWatch.org hosts updated tools to support developing guidelines, training providers, preparing delivery sites and developing demand generation campaigns.
- In addition, Unitaid, the Gates Foundation, and Gilead are supporting LEN implementation research:
- Two Unitaid-funded PrEP implementation projects – ImPrEP in Brazil (led by Fiocruz) and Project PrEP in South Africa (led by Wits RHI) – will be adding LEN.
- Gates is providing support to the Desmond Tutu Health Foundation for a new LEN implementation study, ALIGN, in South Africa.
- Gilead is funding a Phase 4 study, PROPEL, in the United States that will assess real world implementation of LEN and effectiveness outcomes.
- The supply for LEN for these projects is being provided by Gilead.
- The Elton John AIDS Foundation is providing support to Global Fund LEN programming in Kenya, Nigeria, Uganda, and South Africa.
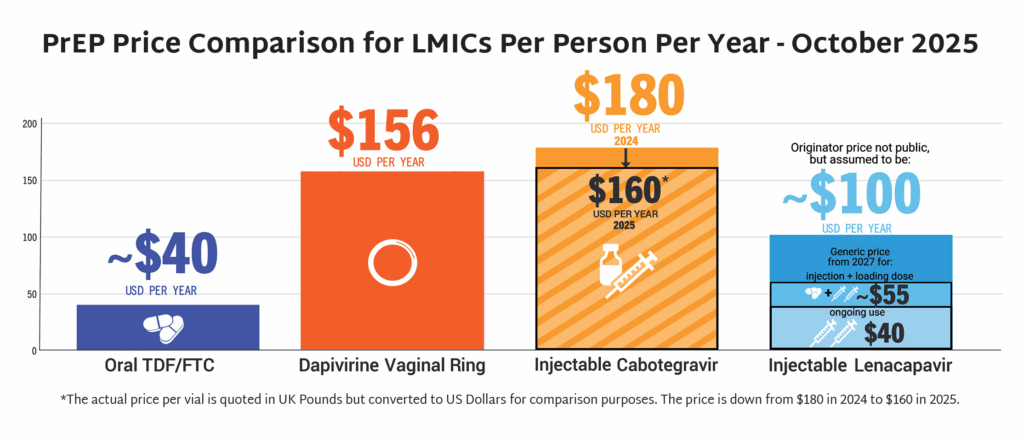
- In September, the Gates Foundation and Unitaid announced partnerships with Hetero Labs and Dr Reddy’s Laboratories, respectively, to achieve a price of $40 USD/person/year for generic injectable LEN. Generic LEN is expected to become available in 2027 in up to 120 countries.
- LEN received WHO prequalification in October, becoming the first product to be prequalified using an expedited process which took just 36 days from the date of submission.
- In the past quarter, LEN has been approved by the European Medicines Agency and submitted in Australia, Canada, and Switzerland.
The Latest R&D in the Prevention Pipeline
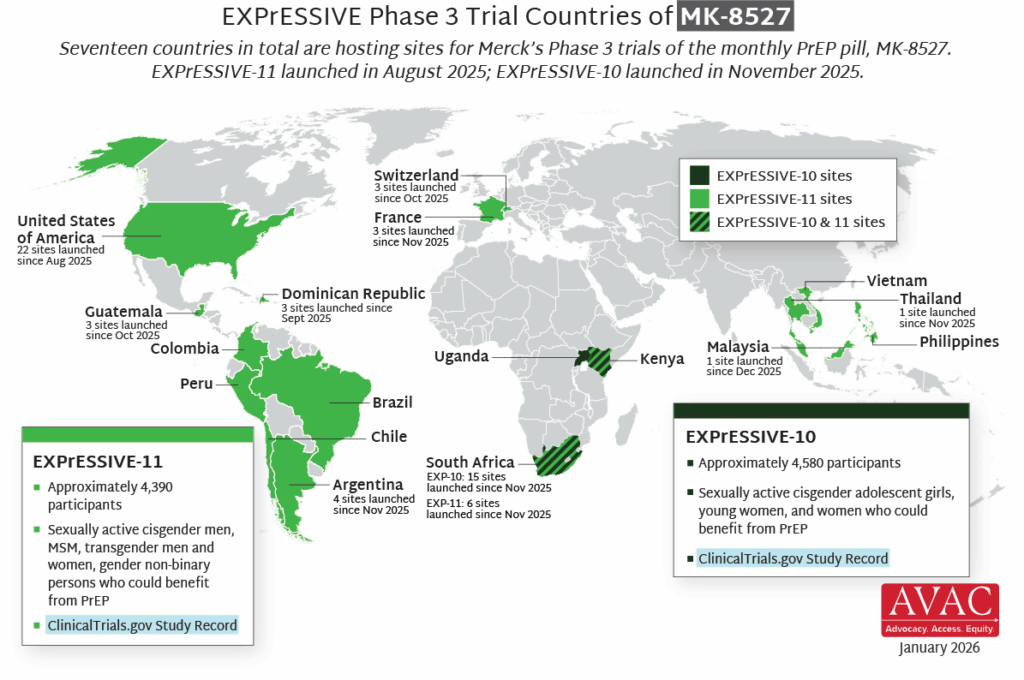
- Seventeen countries are hosting sites for the Phase 3 efficacy trials of a monthly PrEP pill, MK-8527, being developed by Merck (also known as MSD outside of the US and Canada). Merck announced the launch of the Phase 3 trials at IAS 2025 in Kigali. MK-8527 was found to be safe and well-tolerated in Phase 2 clinical trials.
- A long-acting PrEP pill would offer a unique new option to the existing range of PrEP options and could significantly expand use of HIV prevention, especially among young women, key populations, those facing stigma or access barriers, and those that prefer an oral PrEP option over an injectable.
- EXPrESSIVE-10, with funding from the Gates Foundation, is expected to launch in Q4. It will enroll about 4,600 cisgender adolescent girls, young women, and other women who could benefit from PrEP across three countries in Eastern and Southern Africa.
- EXPrESSIVE-11 launched in August 2025, and will enroll about 4,400 cisgender men, MSM, transgender men and women, and gender non-binary persons who could benefit from PrEP in 16 countries. So far, EXPrESSIVE-11 has launched at sites in Dominican Republic, Guatemala, Switzerland, and the US.
- Merck’s commitment to stakeholder engagement to date contributes an important model of Good Participatory Practice (GPP) to the field, by putting global advocates at the forefront of planning for the program and trial design. Merck has expressed a commitment to sustain this vital engagement throughout the program and next steps.
Prevention Playlist
Join
- Do Not Check That Box – Impacts From the Assault on Transgender Communities and DEI + Strategies to Sustain and Rebuild, webinar
- From Courtrooms to Communities: Funding Advocacy to Protect HIV Responses, webinar
- We Declare—Turning The People’s Declaration Demands into Actions and Accountability, webinar
- International Conference on AIDS and STIs in Africa (ICASA 2025), conference
- Subscribe to Global Health Watch
Use
- Now What with Injectable LEN for PrEP?, factsheet
- Guidelines on lenacapavir for HIV prevention and testing strategies for long-acting injectable pre-exposure prophylaxis, guidelines
- Potential Demand for LEN for PrEP, infographic
- Where We Are Now with LEN for PrEP, infographic
- PrEP Price Comparison, infographic
- HIV Prevention Product Overview, infographic
Watch/Listen
- 24 Hours to Save AIDS Research, website & videos
- New WHO Guidance on Lenacapavir for Prevention & HIV Testing for Long-Acting PrEP, webinar
- Next Up: A monthly pill for PrEP?, podcast
- PrEP Implementation — What’s worked and what are we learning, webinar
Read
- AVAC Applauds Agreements to Accelerate Market Development for Lenacapavir for PrEP, statement
- Global Advocates Welcome the Launch of Merck’s EXPrESSIVE Program, statement
- Getting Rollout Right This Time, report
- Key Messages from IAS 2025, newsletter
- Addressing Transgender Erasure in HIV Clinical Trials, publication
- Global Forecast of Long-Acting PrEP Need for Key Populations (2025–2030), report
Avac Event
Beyond Borders
Join ISSTDR, IUSTI, the STI & HIV 2025 World Congress, and AVAC for a special webinar spotlighting speakers who were not about to join the congress due to financial and political barriers. Presenters will share their findings, debate their results, and discuss the work still ahead for the STI field. Don’t miss this opportunity to engage directly with cutting-edge research and the people driving it forward.

Cabotegravir Implementation
Implementation studies completed, ongoing, or planned for cabotegravir as of October 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Dapivirine Vaginal Ring Implementation
Ongoing and planned implementation studies for the dapivirine vaginal ring as of October 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Cabotegravir Volume
Allocation of non-commercial cabotegravir for PrEP supply in low- and middle-income countries, 2023-2026, as of October 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Dapivirine Vaginal Ring Volume
DVR supply available to low- and middle-income countries as of October 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Global Health Watch: US government shuts down, foreign aid funding expires, Jeanne Marrazzo fired from NIH, issue 36
The US government shutdown that began at midnight on October 1 has stalled key public health operations just as the Supreme Court issued a ruling in AVAC v. Department of State that allowed $4 billion dollars in foreign aid to expire, eroding both health and human services and constitutional checks and balances. Meanwhile, Jeanne Marrazzo was officially fired as Director of NIAID, underscoring how politically motivated attacks on science are dismantling the infrastructure that has underpinned decades of progress in HIV prevention and research.
US Government Shuts Down as Federal Funding Expires
At midnight on October 1, US federal funding expired and a government shutdown began after Congress failed to agree to pass a Fiscal Year 2026 (FY26) budget or even a temporary continuing resolution. The shutdown is largely rooted in disagreements over healthcare policy, especially access to healthcare coverage under the Affordable Care Act and Medicaid. The shutdown means “nonessential” federal work is stopped, including many public health operations. Approximately 750,000 federal employees are furloughed, including 40% of Health and Human Services (HHS) staff. CDC disease surveillance is disrupted, many NIH clinical trials are on hold, as is NIH grantmaking and basic research. Many PEPFAR staff have been furloughed as well, but essential “lifesaving” work is continuing.
IMPLICATIONS: The shutdown further destabilizes the US health system and public health infrastructure already weakened by deep cuts. It now comes against the backdrop of a Presidential administration that is using official federal agency websites and social media as propaganda to blame Democrats for the crisis, while the Office of Management and Budget (OMB) director openly threatens mass firings across federal agencies, which is part of the Project 2025 vision he led. For the global health field, the situation highlights how fragile US commitments have become, with billions in foreign aid still frozen and HIV research and prevention programs facing uncertainty. Beyond the immediate loss of services, intimidation tactics on display during this crisis threaten to erode trust and stall science, which will extend beyond the US with consequences for health and human services worldwide.
READ:
- Shutdown Halts Some Health Services as Political Risks Test Parties’ Resolve—KFF
- What happens now that the government has shut down. And, a pricing deal with Pfizer—NPR
- The Supreme Court’s newest decision could make it impossible to end the shutdown—Vox
US Supreme Court Grants Administration Request to Keep Foreign Aid Frozen as Clock Runs Out
Just prior to the US government shutdown, the US Supreme Court (SCOTUS) handed down a decision in AVAC v. Department of State and Global Health Council v. Trump cases, challenging the foreign aid freeze. In a 6-3 decision by emergency order, the court granted the Administration’s request to not spend $4 billion of Congressionally appropriated foreign assistance funds before they expired on September 30, as required by law. This means that, despite the law, and a lower court order, those funds remain unspent. In its statement, AVAC’s Executive Director, Mitchell Warren, warned that the ruling gave the Administration a “free pass” to block disbursement of foreign aid, citing devastation with clinic closures, disruptions in essential services, and lives lost. Moreover, Warren said the ruling undermines constitutional checks on presidential power. “This is beyond foreign assistance; the Court’s decision is a chilling one for anyone who cares about the US Constitution.” In a dissent from the three other Justices, Justice Kagan cautioned that the stakes are too consequential to be decided through emergency orders without full briefing and oral argument and argued that the Administration has not met the stringent standards for such relief.
IMPLICATIONS: While the SCOTUS ruling is not a final judgment, it signals a willingness to allow the President and the Executive branch to withhold funds that Congress has appropriated, potentially whenever it chooses. If unchecked, this precedent could erode the checks and balances that constrain executive overreach and jeopardize not only global health funding but every area of federal spending.
READ:
- Supreme Court Allows Trump to Slash Foreign Aid—New York Times
- The Supreme Court Just Rewrote the Constitution to Give Trump Terrifying New Powers—Slate
- Trump’s USAID pause stranded lifesaving drugs. Children died waiting.—Washington Post
Jeanne Marrazzo Fired from NIAID
Jeanne Marrazzo, former Director of the National Institute of Allergy and Infectious Diseases (NIAID), was terminated on October 1 by Health and Human Services (HHS) Secretary Robert F. Kennedy Jr. after being placed on administrative leave in April. This comes 22 days after Marrazzo and former Fogarty International Center’s director, Kathy Neuzil, filed a whistleblower complaint with the Office of Special Counsel. In her complaint, Marrazzo detailed the Administration’s unlawful cancellation of critical research grants, politicization of science, hostility toward vaccines, and censorship of research. Marrazzo’s lawyers claim that her firing was an act of clear retaliation for her defense of scientific integrity and public health research. Marrazzo succeeded Anthony Fauci as the director of the NIAID in 2023.
IMPLICATIONS: The Office of Special Counsel has been severely weakened by the Administration as have other oversight mechanisms- leaving few pathways for federal scientists and other federal employees to resist politically motivated attacks on research. Holding federal offices accountable to standards of practice that protect the scientific enterprise from political and ideological gamesmanship is essential. Without those standards enforced, decades of progress in science and research are at risk. Marrazzo’s dismissal represents another blow to the independence of US science agencies.
READ:
- After months in limbo, four NIH institute directors fired—Science
- The HHS Officials Being Paid Six Figures to Do Nothing—The Atlantic
- Dr. Jeanne Marrazzo, former Director of NIAID, is Fired by Health and Human Services Secretary Kennedy in Retaliation for Filing Whistleblower Complaint with the Office of Special Counsel—Katz Banks Kumin press release
FOLLOWING: The US May Expand Mexico City Policy / Global Gag Rule
According to the Daily Signal, the US Department of State plans to extend the scope of the Global Gag Rule (often referred to as the Mexico City Policy), which is an executive order that restricts foreign non-governmental organizations (NGOs) from receiving US global health assistance if they provide, refer, or advocate for abortion services. The Global Gag Rule first enacted by the Reagan administration, has been rescinded by every Democratic president and put back in place under every Republican president. Reportedly, the language in this latest iteration of the policy would be expanded to also ban US funding for foreign assistance that promotes “gender ideology” or DEI initiatives. The inclusion of gender affirming care and DEI continues this Administration’s ongoing mischaracterization of existing foreign assistance programs, and attacks on programs that are trans inclusive or seek to address racial and gender disparities.
IMPLICATIONS: This expansion of the Global Gag Rule would fulfill a long-standing conservative goal, explicitly named in Project 2025, to reshape US global public health funding away from evidence-based programs that affirm the needs and dignity of sexual and gender minorities. Turning away from these vulnerable populations further threatens and sets back progress towards ending the global HIV epidemic.
READ:
- EXCLUSIVE: Trump Uses Reagan-Era Policy to Ban Taxpayer-Funded Gender-Transition Surgeries—The Daily Signal
- Trump plans to block funding to groups that promote diversity policies abroad—Politico
What We’re Reading:
- What if NIH had been 40% smaller?—Science
- Viewpoint: What we don’t see about vaccines COULD hurt us—CIDRAP
- Public trust in science has declined since COVID — virologists need to unite around safety standards—Nature
- The damage done—Nature Medicine
- The impact of cuts in the US President’s Emergency Plan for AIDS Relief funding for HIV pre-exposure prophylaxis in sub-Saharan Africa: a modelling study—Lancet HIV
- Deal to lower price of new drug is ‘huge moment of hope’ in global HIV prevention—Healthbeat
- Risks and opportunities’ in US global health strategy—Scidevnet
- America First, World Adrift: Dispatches from a Fractured UN General Assembly—Lights, Camera, Equity!, Jirar Ratevosian substack
- The New America First Global Health Strategy: Four Observations—Think Global Health
- Republicans seek deep cuts to HIV prevention and treatment funding—NBC News
- Will Health Advocates be Classified as Domestic Terrorists?—To End A Plague Substack
- Trump’s scrutiny of nonprofits escalates, with Soros’ OSF at the center—Devex
- Reimagining HIV prevention with artificial intelligence—Lancet HIV
- Meet the AI chatbot that’s talking to young South Africans about sex, HIV and self-harm—Bhekisisa
- As foreign aid falters, can AI step in?—Devex
Resources
- NIH FY26 Contingency plans, National Institutes of Health
- Foreign aid from the United States saved millions of lives each year, Our World in Data
- Tracking State Actions on Vaccine Policy and Access, KFF
Global Health Watch: A generic price for LEN, Future of UNAIDS, UNGA 80, AI for Health, Issue 35
The stakes are high as the US approaches the start of a new fiscal year (FY26) on October 1, currently mired in stalled White House negotiations and a looming government shutdown; the Supreme Court’s pending decision on AVAC’s lawsuit; and the new US “America First” strategy to reshape foreign aid. This issue highlights major global health developments at the UN General Assembly, from debate over the future of UNAIDS to commitments from the Gates Foundation and Unitaid to accelerate access to injectable lenacapavir for PrEP (LEN), alongside new discussions on AI ethics and health.
Gates Foundation and Unitaid Commit to Accelerate Market Development for Lenacapavir for PrEP
The Gates Foundation and Unitaid announced new strategic investments to accelerate the development of, access to and price reduction for generic versions of injectable lenacapavir (LEN), the highly effective six-monthly injection for HIV PrEP. As AVAC’s Mitchell Warren said in its statement, “this could be a transformational moment in HIV prevention if political will, coordination, and further procurement investment meet this moment to deliver LEN with speed, scale and equity to all communities and populations who need and want prevention options.”
IMPLICATIONS: While this progress is encouraging, it is only meaningful if momentum leads to real access. These agreements get LEN closer to the $40 per person per year price of daily oral PrEP for many, but not all, low and middle-income countries, and hopefully will accelerate large scale programs by 2027. AVAC’s publication, Now What with Injectable LEN for PrEP How to Translate Ambition into Accelerated Delivery and Impact, includes forecasts demonstrating that instead of 2 million people in three years that is currently being planned by the Global Fund and PEPFAR with initial supplies from Gilead, the world could reach at least 1.5 million people in just one year, rising to at least five million people per year by 2030. These numbers suggest what is possible and what is necessary to accelerate access, achieve real impact, build a sustainable market, and drive prices down even further.
READ:
- Gates Foundation announcement
- Unitaid announcement
- Twice-yearly HIV prevention drug to be offered at new, low cost—The World
- Philanthropies Strike a Promising Deal to Turn Back H.I.V.—New York Times
- New partnerships bring price parity between lenacapavir and oral PrEP—Devex
- AVAC Applauds Agreements to Accelerate Market Development for Lenacapavir for PrEP—AVAC statement
- Joint Statement: Activists Demand $40-a-Year Generic Price for Breakthrough HIV Prevention Drug Be Made Available to All Low- and Middle Income Countries—TAG, Public Citizen, Health Gap
UN General Assembly Updates
The UN General Assembly (UNGA 80) kicked off this week in New York with global health taking center stage. The US began the rollout of its new “America First Global Health Strategy,” which shifts toward bilateral aid models. Meanwhile, HIV innovation and access, especially for lenacapavir, are being debated in side events as delegates push for clarity on price, procurement, and equity. The WHO is accelerating its health agenda on noncommunicable diseases (NCDs) and mental health and will help lead discussions on a new global declaration.
In its annual Goalkeepers event, the Gates Foundation announced its $912 million commitment in the current round of replenishment to the Global Fund for AIDS, TB and Malaria. The Foundation also recognized ten champions in global health, including AVAC’s partner Jerop Limo, a leading HIV and sexual and reproductive rights activist from Kenya.
Next week, a new topic will take center stage at the UNGA’s high-level meetings: inclusive and accountable governance of artificial intelligence (AI). Ethics and equity will be central to deploying responsible AI for health, with advocates emphasizing that progress must be measured not only by rollout speed but by how well it protects patient privacy and addresses real-life challenges and needs. Development of AI governance to shape how digital tools are designed, regulated, and financed is a key part of the next generation of HIV and health programming.
READ:
- Bill Gates pledges $912 million to global disease fight, urges governments to step up—Reuters
- UNGA80 reporters’ notebook: Day 4—Devex
- Global Health Multilateralism Without the United States: What Comes Next?—Think Global Health
- The Ethics of AI-Driven Health Projects in Africa—Think Global Health
United Nations Secretary-General Proposes to “Sunset” UNAIDS
The UN Secretary-General shared a proposal in his new UN80 progress report to “sunset” UNAIDS by the end of 2026 and fold its mandate into broader UN structures in the face of funding cuts. The NGO delegation to the UNAIDS Programme Coordinating Board (PCB) strongly opposes this move, and was joined by nearly 800 civil society organizations warning that dismantling UNAIDS now would undermine leadership, coordination and accountability at a time of escalating funding cuts, growing inequalities, and service disruptions. UNAIDS was originally created to bring coherence across 11 UN agencies, avoid duplication, and ensure that communities most affected by HIV had a formal voice at the table. The plan endorsed by the UNAIDS PCB would downsize the Secretariat, embed staff in select UN Resident Coordinator offices and relocate programmatic expertise to regional hubs to align with the UN80 “Shifting Paradigms” vision of a more integrated, coherent UN system. As UNAIDS reminded all stakeholders in its statement, it is member states and governing bodies who should determine the way forward on how UN80 reforms are implemented.
IMPLICATIONS: Achieving the UNAIDS goal of ending AIDS by 2030 depends on many factors, including clear accountability; knowing which UN agencies retain their strengths; ensuring they have the resources to deliver; and safeguarding coordination. Sustaining trusted partnerships with civil society and continuing to prioritize equitable rights-based programs, which have been central to UNAIDS’s role for two decades, will also be essential, and all of this risks being undermined if UNAIDS’ functions are dispersed without a coherent strategy. This debate marks a critical inflection point for how the global community organizes and funds the HIV response going forward.
READ:
- With the HIV Response in Crisis, UNAIDS Must Not Be Sunset in 2026—NGO Delegation to the UNAIDS PCB statement
- UN proposes closing UNAIDS in 2026 as funding cuts bite—Reuters
- The world cannot afford to lose UNAIDS—Erasing 76 Crimes
Africa CDC Announces Grant to Support Local Drug and Vaccine Manufacturers
The Africa CDC plans to invest approximately $3.2 billion dollars to support the development of local drug and vaccine manufacturing across the continent. The initiative includes funding and grant support for African manufacturers. This grant aims to reduce dependence on imported pharmaceuticals by strengthening domestic production and establishing a pooled procurement mechanism to guarantee market demand.
IMPLICATIONS: This move could be a turning point for health sovereignty in Africa, offering the promise of more reliable supply chains, lower costs, and greater independence from external donor trends. But it also presents real challenges: scaling production to meet global standards, navigating regulatory harmonization, and maintaining quality assurance and sustainability will be essential. If successful, it could shift power in global health — giving African countries more leverage in pricing and access negotiations for prevention tools such as ARVs and vaccines, while reducing vulnerability during global supply disruptions.
READ:
- Africa CDC Unveils $3.2 Billion Plan to Transform Vaccine and Drug Production—Addis Insight
- Africa CDC earmarks Shs11 trillion to boost local drug manufacturing—The Monitor (Uganda)
What We’re Reading
- CDC Backs Twice-Yearly Injectable for HIV Prevention—MedPage Today
- Public Health Associations Call on RFK Jr. to Resign as HHS Secretary, Healthcare Innovation
- Gold standard science requires gold standard scholarship, Science
- U.S. court orders NIH to restore killed grants to California researchers, Science
- Censorship returns to the CDC. At least 22 webpages are down.—Inside Medicine Substack
- RFK Jr cancelled mRNA research — but the US military is still funding it—Nature
- How ex-USAID staffers turned crisis into action and mobilized $110M—Devex
- 90% of rich countries’ global health R&D goes to domestic institutions—Devex
- Unpacking the Role, Nature, and Need to Sustain Robust Community Responses in Global Systems—Journal of Infectious Diseases
- PrEP4All’s Jeremiah Johnson Responds to CDC Guidelines on Lenacapavir, Calls for a A National PrEP Program for Equitable Uptake—PrEP4All
- NIH outlines new system for awarding research grants to foreign scientists—STAT