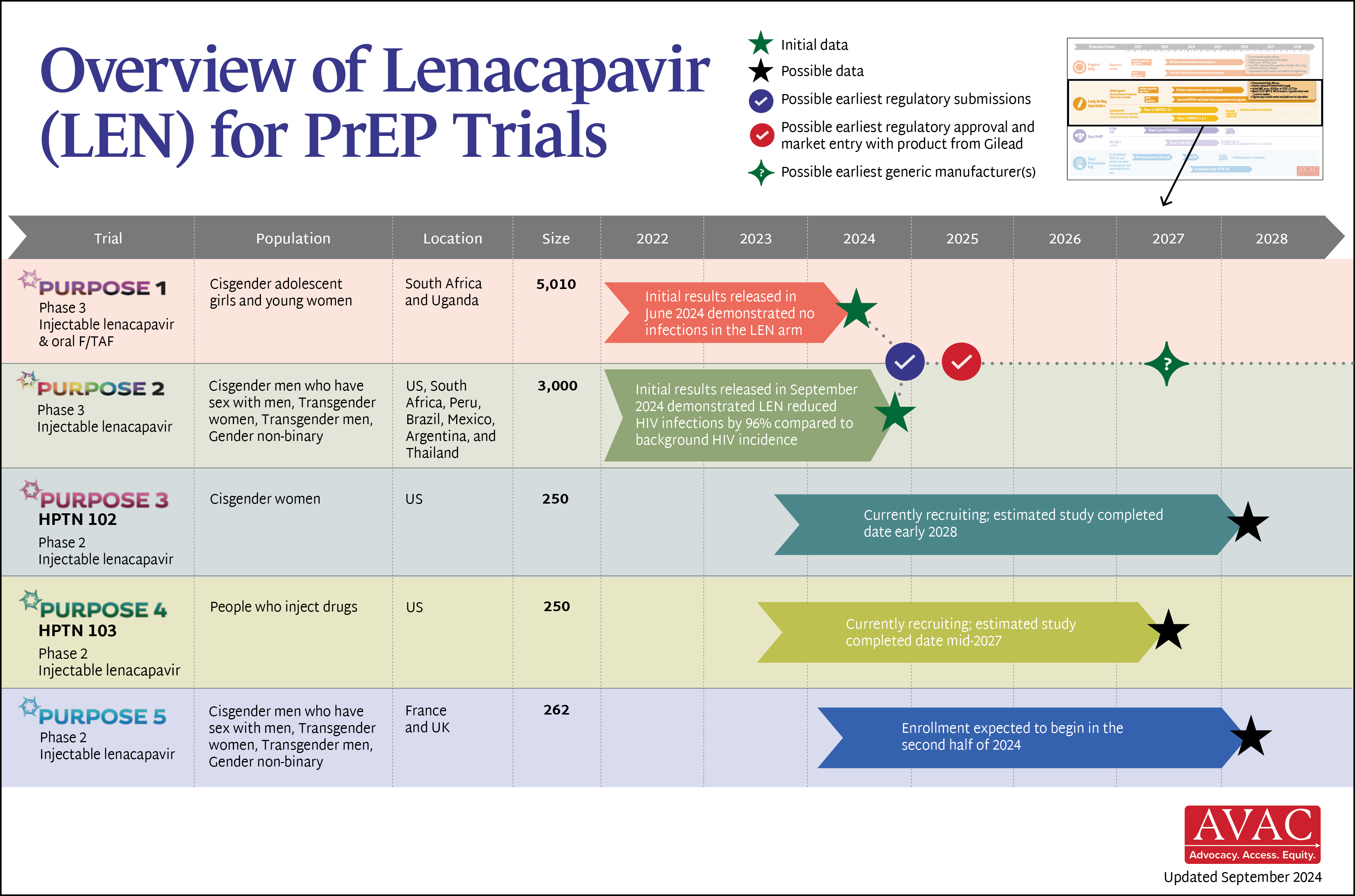
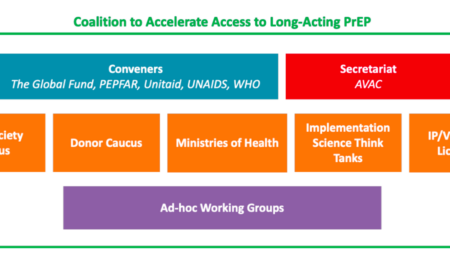
report
Gears of Lenacapavir for PrEP Rollout
This document outlines a focused plan for LEN for PrEP rollout over the next few years, specifying priorities by stakeholder and evaluating volume and pricing strategies. The report as a whole details a coordinated response to this historic opportunity to ensure rapid implementation, equitable access, and sustainable impact.