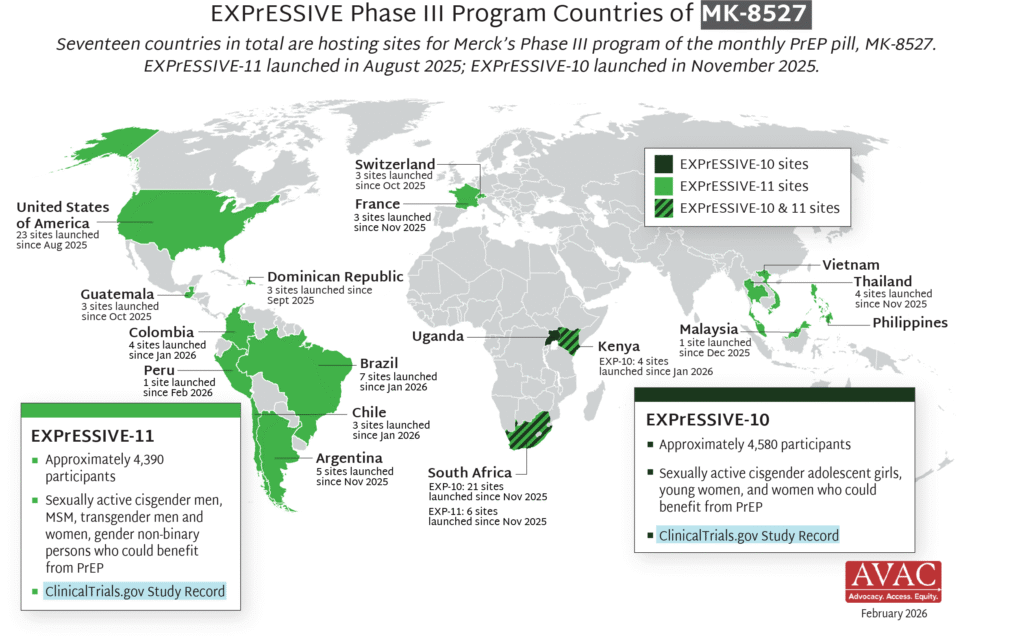
Seventeen countries are hosting sites for the Phase III efficacy trials of a monthly PrEP pill, MK-8527, being developed by Merck (also known as MSD outside of the US and Canada). Merck announced the launch of the Phase III trials at IAS 2025 in Kigali. MK-8527 was found to be safe and well-tolerated in Phase II clinical trials.
Was this content helpful?
Tell us how we can improve the content.
Was this content helpful?
Thank you for your feedback!