Resources
AVAC’s Resource Database contains educational and advocacy materials covering a wide range of issues on biomedical prevention of HIV, STIs, COVID-19 and emerging health threats—from research to rollout.
To search for clinical trials and detailed information on products in development, visit our Prevention Research & Development Database

Results
showing 1-10 of 170
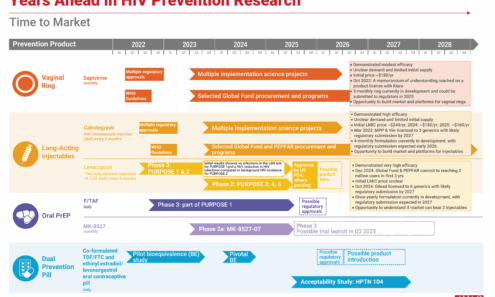
Years Ahead in HIV Prevention Research: Time to Market
This timeline shows the potential time points when the next-generation of HIV prevention options might find their way into new programs.
Prevention Option:
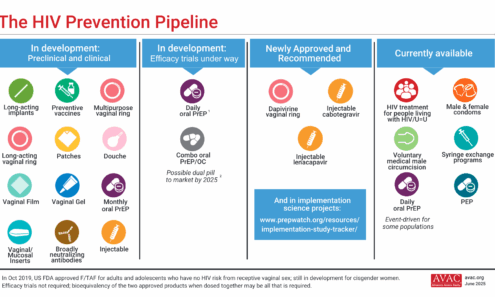
The HIV Prevention Pipeline
This graphic shows currently available options for HIV prevention, newly approved and recommended treatment, and those in development.
Prevention Option:
From The Lab To The Jab: Lessons learned and what’s next in HIV vaccine research
On 3 June 2024, AVAC hosted a webinar highlighting its Lab to Jab issue briefs on research and development, production and equitable global access to vaccines. Platforms, Not Pathogens Panelists stressed an intentional, rather than incidental...
Prevention Option:
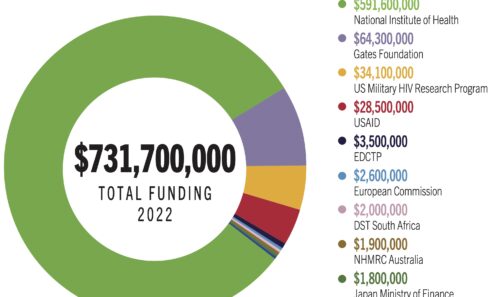
Top Vaccines Funders
Total US dollars invested by each of the top 10 global funders of vaccine research in 2022.
Prevention Option:
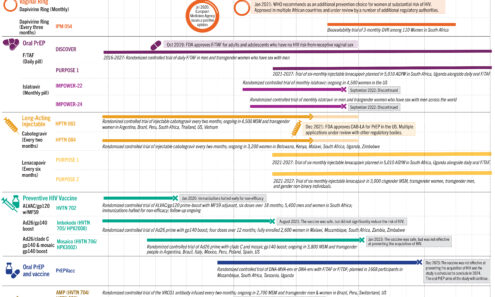
The Years Ahead in Biomedical HIV Prevention Research
This graphic shows the updated status of large-scale prevention trials through the end of 2024.
Vaccine Development History
A graphic showing the duration between discovery of the microbiologic cause of selected infectious diseases and the development of a vaccine.
Prevention Option:

HIV Vaccine Research
HIV vaccine research is a multifaceted endeavor that requires collaboration across many disciplines to develop a vaccine that protects people from HIV infection. The graphic outlines the different areas of research that are being pursued, including vaccine platforms, immune system research, clinical research capacity, and community engagement.
Prevention Option:

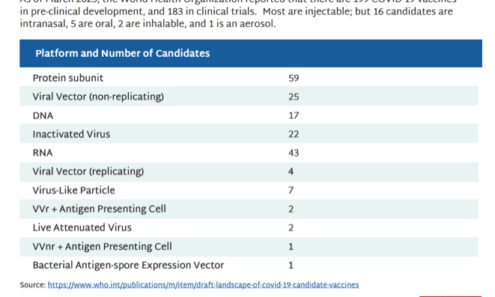
Getting the COVID-19 Vaccines We Need
As of March 2023, the World Health Organization reported that there are 199 COVID-19 vaccines in pre-clinical development, and 183 in clinical trials. Most are injectable; but 16 candidates are intranasal, 5 are oral, 2 are inhalable, and 1 is an...
Prevention Option:
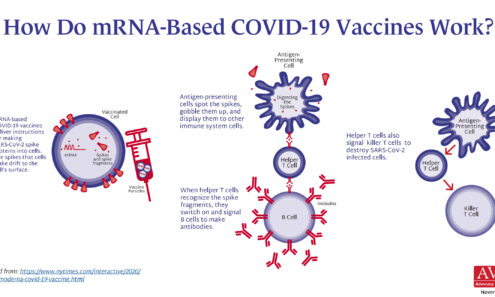
How Do mRNA-Based COVID-19 Vaccines Work?
A detailed graphic showing the biological mechanisms by which mRNA vaccines work.
Prevention Option:
Other mRNA Vaccines in Development
mRNA technology is currently being studied for many different uses.
Prevention Option:

showing 1-10 of 170