Resources
AVAC’s Resource Database contains educational and advocacy materials covering a wide range of issues on biomedical prevention of HIV, STIs, COVID-19 and emerging health threats—from research to rollout.
To search for clinical trials and detailed information on products in development, visit our Prevention Research & Development Database

Results
showing 1-10 of 216
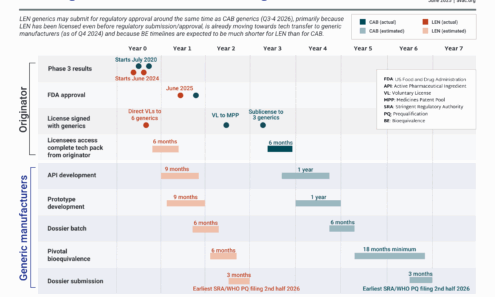
LEN Generics — Can we go faster?
The timeline for generic LEN for PrEP to come to market is expected to be significantly shorter than for CAB for PrEP. Bioequivalence (BE) testing for LEN, which demonstrates a generic product works in the body in the same way as the originator product, is likely to be six months, vs. the 18 months for CAB for PrEP, because of differences in the drug formulation.
Prevention Option:
Protect Federal Funding for HIV, TB, and STI Research and Prevention at the National Institutes of Health
AVAC and 627 organizations, institutions, researchers, clinicians, public health advocates and stakeholders submitted a written letter to the Senate HELP Committee urging lawmakers to reject the cuts to NIH funding for HIV, TB, and STI research and highlighting the impact of these cuts on lifesaving innovation and research infrastructure.
Prevention Option:
Advocates’ Guide: Understanding the President’s Proposed Fiscal Year 2026 (FY26) Budget and Its Implications for Science, Research and Global Health
The US administration’s proposed Fiscal Year 2026 (FY26) budget marks a sweeping rollback of federal investment in health, research, and global development. For advocates, researchers, and implementers, this proposal demands urgent attention and action.
Prevention Option:
Worldwide Prevention, Shared Protection
This Issue Brief describes the impacts of the elimination and reduction of funding that supports sexually transmitted infection (STI) research, testing, and prevention programming. This funding is critically important as STI rates continue to increase globally with more than 1 million curable STIs, including chlamydia, gonorrhea, syphilis, and trichomoniasis, acquired every day. Without appropriate testing, treatment, and prevention programs, there is a risk that STI rates will continue to increase leading to more cases of infertility, pelvic inflammatory disease, and cancers.
AVAC and FAPP Written Statement: US Senate Hearing on Biomedical Research
AVAC and GAPP submitted written testimony for the US Senate Appropriations Subcommittee’s April 30th hearing, “Biomedical Research: Keeping America’s Edge in Innovation.”

Research Matters Advocacy Toolkit
This toolkit for researchers shares key messages, practical advocacy guides, and resources to help move our collective efforts forward.
Prevention Option:

Lawsuit Wins and What’s at Stake
On February 10, AVAC led other organizations to sue the US government including the President, the US State Department and USAID, seeking emergency relief from an Executive Order that inhumanely froze all funding for foreign assistance. This case may well help to determine the future of foreign assistance, executive overreach, and the role of evidence, facts, and values in US policy.
AVAC’s Executive Director, Mitchell Warren and Public Citizen Litigator, Lauren Bateman explain these lawsuits and why they matter.
The Role of PEPFAR in HIV Prevention
AVAC Senior Program Manager for Policy, John Meade Jr., describes PEPFAR’s historic legacy and strongly argues for its continued importance in the face of attacks by the new US administration. This piece appears in The Broadsheet, the magazine published by the Congressional Black Caucus Health Braintrust.

Impact of PEPFAR’s Stop Work Order on PrEP
The impact of the stop-work order on PrEP is expected to be severe. These slides reflect the results of an analysis drawing on key informant interviews with representatives of Ministries of Health and PrEP implementers between 27 January 2025, when...
Prevention Option:
PxWire Volume 15, Issue No. 1
In this special edition of Px Wire, AVAC is going beyond a quarterly update of biomedical HIV prevention. We look at how the new US Administration’s attack on global health can be expected to devastate HIV prevention, including the capacity to deliver existing PrEP options, the scale up of new PrEP products, and the paralyzing impact on research and development.
Prevention Option:

showing 1-10 of 216