Resources
AVAC’s Resource Database contains educational and advocacy materials covering a wide range of issues on biomedical prevention of HIV, STIs, COVID-19 and emerging health threats—from research to rollout.
To search for clinical trials and detailed information on products in development, visit our Prevention Research & Development Database

Results
showing 1-10 of 565
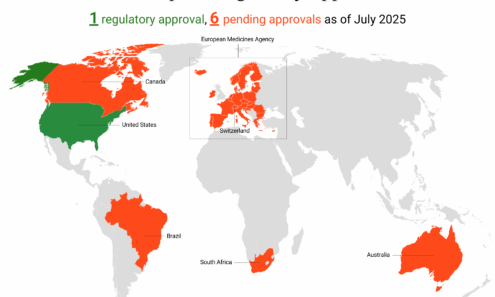
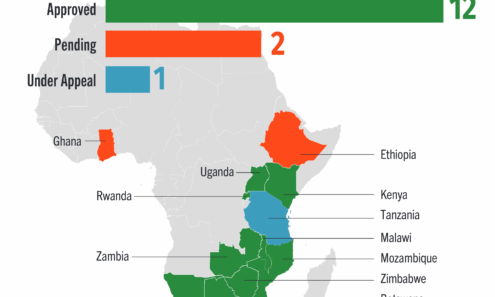
Lenacapavir Regulatory Approval
Regulatory approvals, pending decisions, and appeals as of July 2025, including US Food and Drug Administration approval. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard...
Prevention Option:
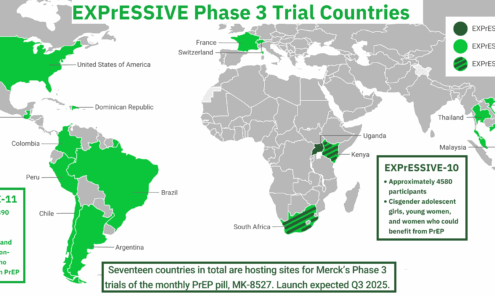
EXPrESSIVE Phase 3 Trials
Seventeen countries are hosting sites for Merck’s trials of a monthly pill for PrEP. Launch is expected in Q3 2025. This graphic shows where these trials are taking place.
Prevention Option:
HIV Research on Pause
This presentation, delivered by AVAC’s Executive Director, Mitchell Warren at IAS 2025, shares a sobering picture of the sweeping changes to science, global health and particularly, HIV R&D since January 20, 2025. It outlines the impact of foreign aid cuts, NIH grant terminations, and policy shifts and shares a vision for the future.
Prevention Option:
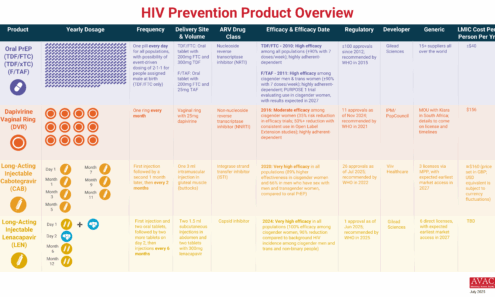
HIV Prevention Product Overview
The graphic provides an overview of PrEP products currently available and in late-stage clinical trials.
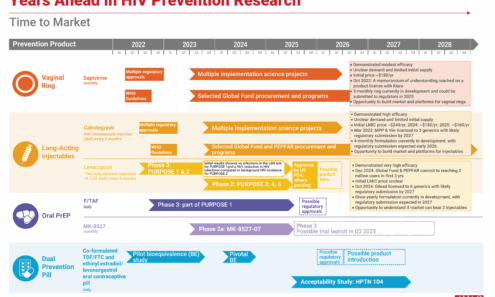
Years Ahead in HIV Prevention Research: Time to Market
This timeline shows the potential time points when the next-generation of HIV prevention options might find their way into new programs.
Prevention Option:
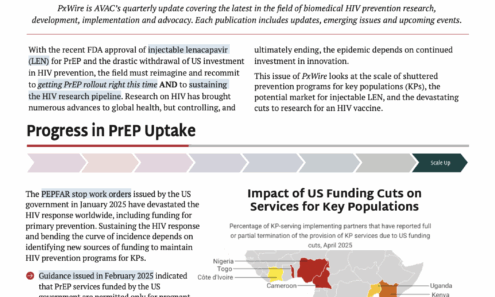
PxWire Volume 15, Issue 3
This issue of PxWire looks at the scale of shuttered prevention programs for key populations, the potential market for injectable LEN, and the devastating cuts to research for an HIV vaccine.
Prevention Option:
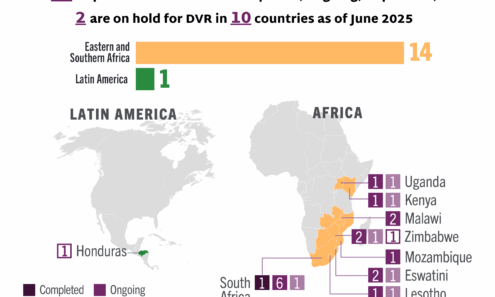
Dapivirine Vaginal Ring Implementation
Ongoing and planned implementation studies for the dapivirine vaginal ring as of June 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Prevention Option:
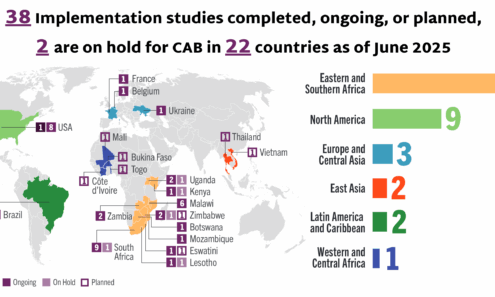
Cabotegravir Implementation
Implementation studies completed, ongoing, or planned for cabotegravir as of June 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Prevention Option:
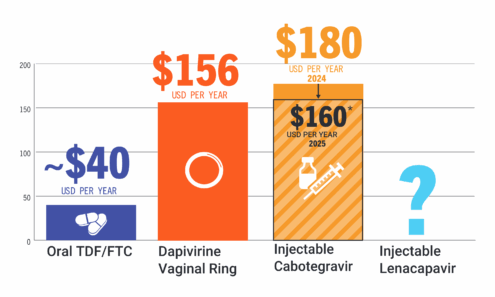
PrEP Price Comparison
Comparing the annual price of oral TDF/FTC vs. the dapivirine vaginal ring and injectable cabotegravir. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Prevention Option:
Dapivirine Vaginal Ring Regulatory Approval
Regulatory approvals, pending decisions, and appeals as of June 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Prevention Option:
showing 1-10 of 565