Resources
AVAC’s Resource Database contains educational and advocacy materials covering a wide range of issues on biomedical prevention of HIV, STIs, COVID-19 and emerging health threats—from research to rollout.
To search for clinical trials and detailed information on products in development, visit our Prevention Research & Development Database

Results
showing 1-10 of 968
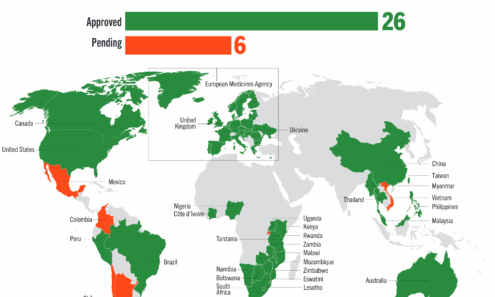
Dapivirine Vaginal Ring Regulatory Approval
Regulatory approvals, pending decisions, and appeals as of September 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Prevention Option:
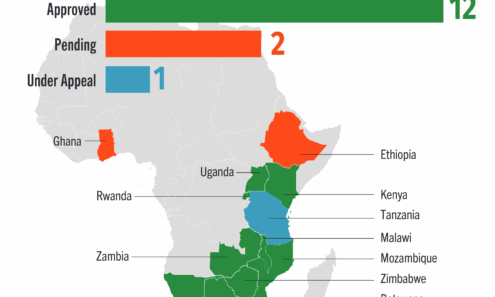
Cabotegravir Regulatory Approval
Regulatory approvals and those pending for cabotegravir as of September 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Prevention Option:
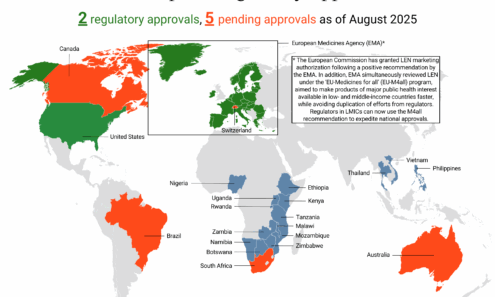
Lenacapavir Regulatory Approval
Regulatory approvals, pending decisions, and appeals as of September 2025, including US Food and Drug Administration approval.
Prevention Option:
The Real-World Impact of Defunding STI Research
The US presidential administration’s funding cuts and policy shifts are reshaping the public health landscape in profound ways. While many of these changes have drawn significant media attention, the impact on sexually transmitted infection research and prevention has remained largely overlooked, though the consequences are dire, writes AVAC’s Alison Footman writes in TheBodyPro.
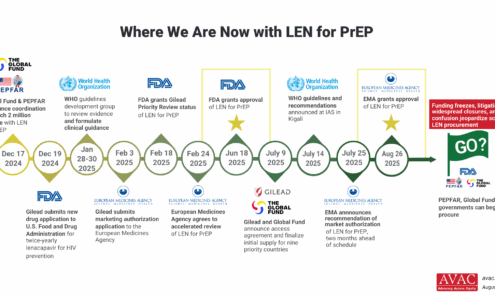
Where We Are Now with LEN for PrEP
The chaos in foreign assistance programs (including discontinuation of major PrEP programs), cuts in staffing and new demands on donor commitments will make decisions on the procurement of LEN for PrEP more complex and uncertain.
Prevention Option:
Global Advocates Welcome the Launch of Merck’s EXPrESSIVE Program
Known as the EXPrESSIVE program, the drug maker Merck has two trials testing a monthly pill for PrEP. Merck is committed to stakeholder engagement, putting global advocates at the forefront of planning for the program. Numerous organizations and advocates commend Merck’s dedication to hearing from the community and shared this statement.
Prevention Option:
Topic:

Next Up: A monthly pill for PrEP?
This episode features Merck Distinguished Scientist Rebeca Plank and AVAC’s Regional Manager for Research Engagement Grace Kumwenda. They explain why a monthly pill could be so important to HIV prevention and how GPP is shaping the design and rollout of the trials.
Prevention Option:

HIV Prevention R&D at Risk
AVAC’s analysis of the impact of US Government funding cuts, terminated projects, and other policy changes on the HIV prevention research and development (R&D) pipeline, and on HIV research broadly.

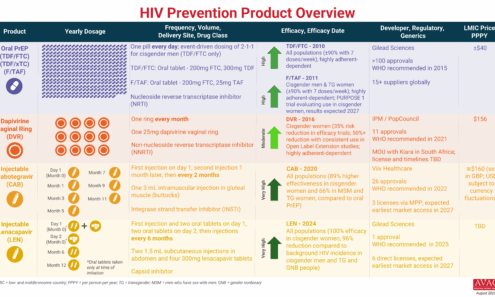
HIV Prevention Product Overview
The graphic provides an overview of PrEP products currently available and in late-stage clinical trials.
STIWatch Quarterly Newsletter
As many global health fields reassess their reliance on US government funding for research and development, the STI field—already underfunded and reliant on alternate donors—now faces even greater uncertainty. In this newsletter, we share a new STI resource for advocates and highlight the top issues we’re monitoring as events continue to unfold.
showing 1-10 of 968