Developing a rich pipeline of options for HIV prevention is essential and must be guided by community priorities that define what products are needed and supported through R&D.
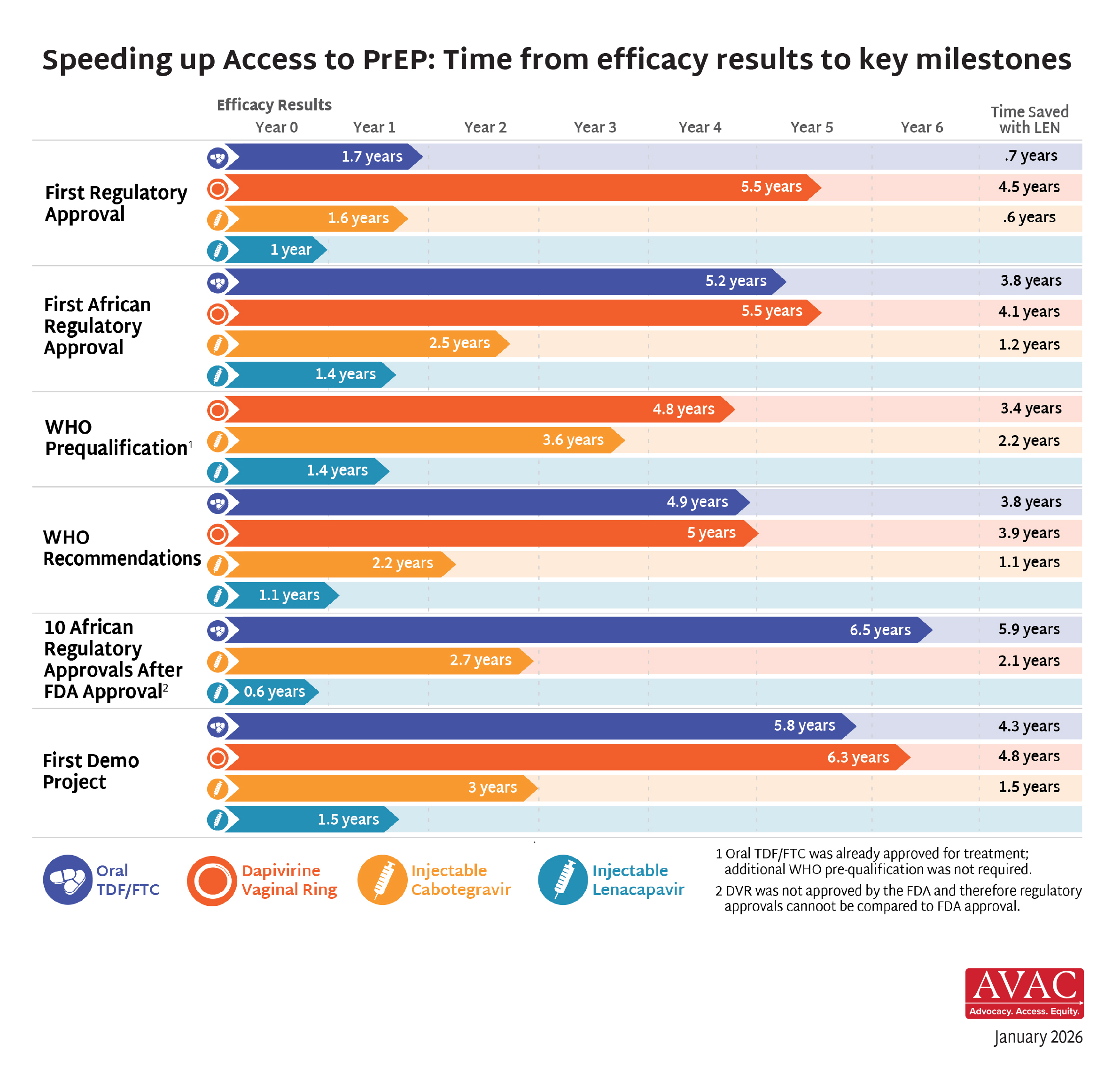
Once approved, interventions typically become widely available in wealthy countries within a few years time. But scaling up new options in lower and middle-income countries lags for years, even decades, with devastating effects on global health, individual lives, and the global effort to end the epidemic.
AVAC’s work supports:
- Development of timely, effective and affordable prevention options
- Including community needs and perspectives in R&D
- Compiling and analyzing market data
- Producing data, lessons learned, tools, and resources that support product introduction
- Collaborating with partners to get products faster to market and to scale
- Sharing and translating information, including around choice in the development and rollout of new products
- Advocating for the integration of HIV services with sexual and reproductive health
AVAC partners closely with Access Bridge to inform and accelerate product introduction and access. Access Bridge is an independent, Kenya-based not-for-profit rooted in African leadership and global collaboration that was incubated at AVAC. Access Bridge advances timely, sustainable, and equitable introduction of and access to HIV prevention, SRH and related health products.