report
PxWire Volume 16, Issue 1
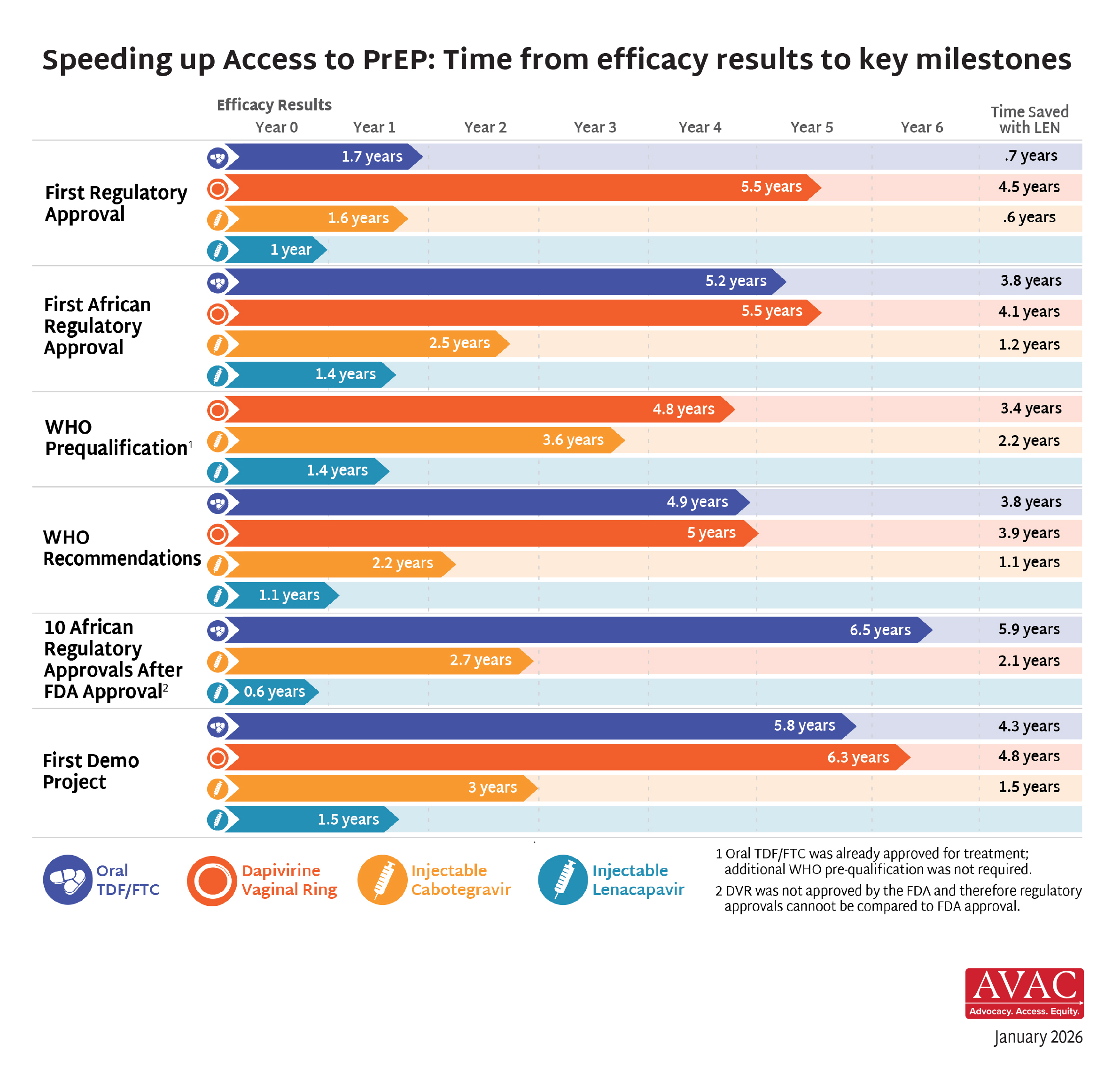
This issue showcases the status of access to oral PrEP with the best data available since the US foreign aid freeze disrupted PEPFAR operations. Stakeholders are digging deep to meet the moment, which includes an unprecedented opportunity to drive down HIV incidence with the rollout of lenacapavir. Lastly, our update on the HIV vaccine R&D pipeline documents an evolving and diverse portfolio of products, which remains essential for a durable end to HIV as a global health threat.