As overall sexually transmitted infections (STI) rates continue to rise globally, STI diagnostic and vaccine research and development (R&D) investments remain underfunded and neglected compared to other infectious diseases, a new AVAC report finds.
This report, Sexually Transmitted Infections: A Review of the 2022 Vaccine and Diagnostic Research and Development Pipeline and Investments, examines disbursements by the U.S. National Institutes of Health (NIH) and the Bill & Melinda Gates Foundation—the largest investors across a vast range of global health R&D areas—and is one of few reports to track funding trends in vaccine and diagnostics R&D, and pipeline investments for some of the most common STIs, including chlamydia, genital herpes, gonorrhea, hepatitis B, human papillomavirus (HPV), syphilis, and trichomoniasis.
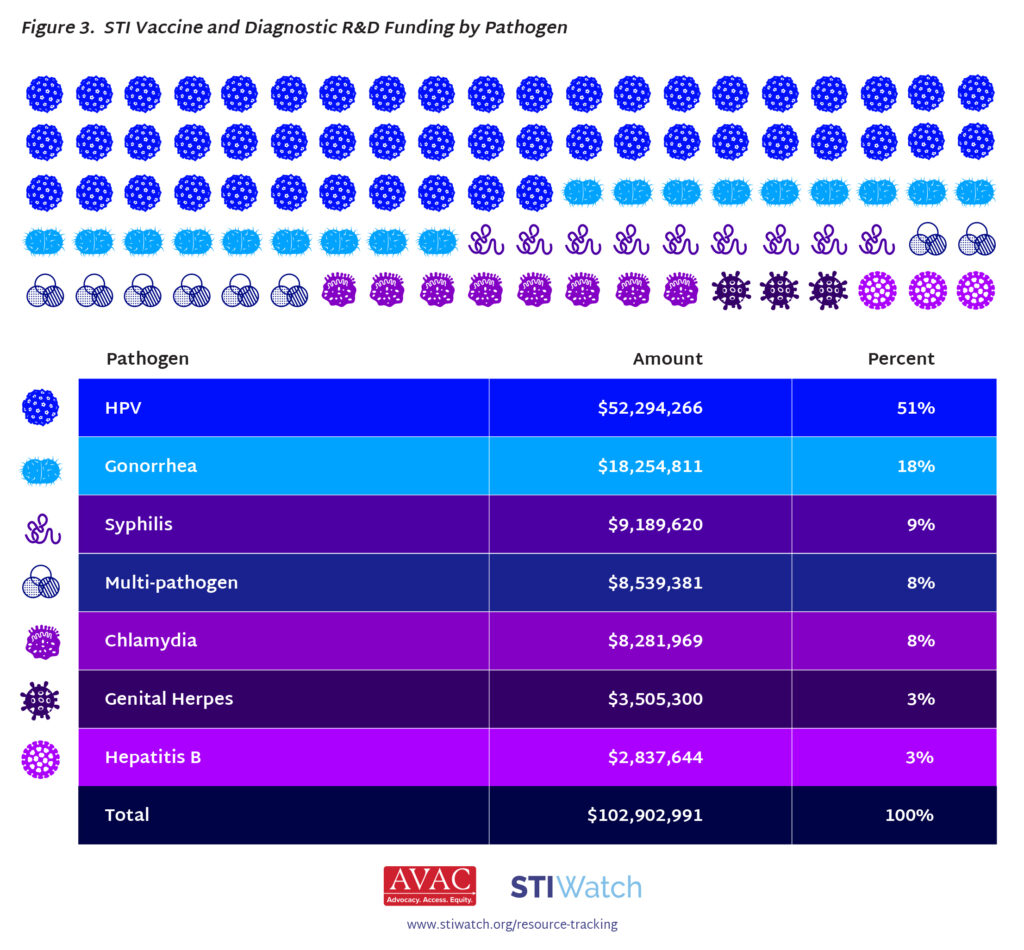
It found that leading philanthropic and public funding for STI vaccine and diagnostic R&D totaled just US $103 million in 2022 with most of the funding coming from the NIH, at US $78 million, approximately 76 percent of total reported funding disbursements.
“We’re seeing the gap widen—as the global burden of STIs continues to increase, funding for R&D tools to reduce this burden lags behind,” said Alison Footman, PhD, senior program manager for STIs at AVAC and lead author of the report. “If we’ve learned anything from the HIV and COVID-19 epidemics, it’s that we need to get ahead of transmission by prioritizing the health of people and communities and committing the resources needed to develop user-centered diagnostics, treatments and prevention methods. Point-of-care tests that are effective, affordable, and user-friendly, and vaccines to prevent and treat STIs are on the horizon but will only be developed and delivered if money is made available with urgency and scale.”
Of the total US $103 million in STI vaccine and diagnostic R&D funding reported, only seven percent (US $6.8M) was dedicated to diagnostic research—a much lower amount than vaccine research, which was funded at US $93 million or 90 percent of the total funding. The remaining 3 percent was directed towards projects that examined both vaccine and diagnostic R&D. As with vaccine R&D, the NIH accounted for most of the diagnostic R&D investments (73 percent).
“Many STI testing programs rely on patients to present with symptoms. But considering that most STIs are asymptomatic, this approach is missing the mark,” Footman added. “We need to be investing in tests for STIs that will improve detection, and therefore limit the time between test and treatment, and hopefully prevent transmission. The overall STI R&D investments are already too little overall, and just seven percent of total funding dedicated to diagnostics is not commensurate with the public health need.”
Other Key Findings
- Six institutes and centers within the US NIH contributed the bulk of funding (US $78 million) toward STI diagnostic and vaccine R&D.
- 59 organizations were funded worldwide to conduct STI R&D; of those, 78 percent (n=46) were located in the US.
- Institutions in South Africa and Zambia were the only African countries where local organizations were funded to conduct STI vaccine and diagnostic research. In total, US $814,279 was provided directly to these two countries, representing less than 1 percent of total funding spent.
- By pathogen, most funding (51 percent) was dedicated to HPV R&D, with gonorrhea and syphilis rounding out the top three pathogens funded.
- US $50 million was spent on HPV vaccine R&D, $18 million on gonorrhea vaccine R&D, $9 million on syphilis vaccine R&D, $8 million on chlamydia vaccine R&D, $3.5 million on genital herpes vaccine R&D, and $2.8 million on Hepatitis B vaccine R&D.
- NIH spending accounted for most STI diagnostic R&D investments (73 percent).
- Of diagnostic funding available, 66 percent was dedicated to multi-pathogen research, 33 percent towards HPV, and 1 percent towards hepatitis B. (Multi-pathogen diagnostic projects included research on syphilis, gonorrhea, genital herpes, and chlamydia).
The report also includes spotlights on DoxyPEP; on WHO’s new reports and manuals that provide information on diagnostics currently available to guide development; and on seven AVAC partners in East and Southern Africa who received funding to conduct projects on community needs to prevent, detect, and treat STIs. These advocacy projects helped build a stronger advocacy movement to improve funding and commitments in and around STI vaccines and diagnostics.
For years, AVAC has been tracking investments and funding trends in HIV prevention and cure R&D.
This new report on STI vaccine and diagnostic R&D is intended to help decision-makers and advocates identify current funding investments, opportunities, and gaps. Tracking investments over time can also demonstrate the effects of public policies and guidelines, such as WHO’s STI Vaccine Roadmap, on funding decisions and inform research priorities.
For more information on the state of STI vaccines and diagnostics R&D, visit STIWatch.org, or get in touch at avac@avac.org.