This week marks one year since AVAC and the Global Health Council filed their lawsuits over the sweeping foreign assistance freeze — cases that challenged the Administration’s withholding of Congressionally appropriated funds and underscored the stakes for global health and HIV programs. That broader dispute over executive power and federal spending continues to reverberate, as four US states sued the administration this week over its decision to withhold $600 million in public health grants supporting disease surveillance, emergency preparedness, vaccination, and HIV prevention. This Global Health Watch issue also examines ongoing signs of erosion in vaccine confidence and regulatory stability and calls for early global access planning for Merck’s investigational monthly oral PrEP candidate.
Four US States Sue HHS Over $600 Million in Public Health Grants
Last week, the US administration announced it would withhold approximately $600 million in previously allocated public health grants for disease surveillance, emergency preparedness, vaccination programs and HIV prevention from states it argues are not aligned with administration priorities. In response, these states, which are led by Democrats: California, Colorado, Illinois and Minnesota, filed a lawsuit against the US Department of Health and Human Services (HHS), seeking to block the cuts. The funding is primarily distributed through the US Centers for Disease Control and Prevention (CDC) and the states argue that the administration cannot retroactively impose new conditions on congressionally appropriated funds or withhold them based on policy disagreements.
IMPLICATIONS: This case mirrors broader disputes over executive authority and federal spending, including AVAC’s lawsuit, that have surfaced repeatedly over the past year. Beyond the immediate budget impact, the decision to withhold public health funds risks destabilizing prevention and preparedness systems that rely on sustained, predictable financing. For HIV and other infectious diseases, interruptions in surveillance, community outreach, and prevention programming can quickly translate into increased transmission and weakened response capacity. While $600 million in funding is on the line, so is the principle that public health infrastructure cannot function effectively if appropriated funds are subject to shifting political leverage.
READ:
- Four States Sue Administration Over Loss of Public Health Funds—New York Times
- Four states sue Trump administration over cuts to public health funding—Reuters
- ‘Sanctuary’ States Get Judge to Pause Trump’s Public Health Cuts—Bloomberg
- Save HIV Funding Campaign Denounces Cancellation of Vital HIV and STI Funding, Urges the Administration to Immediately Restore Grants
Vaccine Confidence Continues to Be Undermined
Vaccine confidence continues to be undermined across multiple fronts this week. The US Food and Drug Administration (FDA) declined to review Moderna’s mRNA seasonal influenza vaccine candidate, raising questions about the regulatory pathway and timeline for review and introduction of new flu products. At the same time, the American Medical Association (AMA) announced its plans for its own independent vaccine safety and effectiveness review initiative, which signals growing concern about trusted scientific assessment processes. And a survey found declining public perceptions of the safety of COVID-19, influenza, and MMR vaccines, underscoring persistent erosion of vaccine confidence.
IMPLICATIONS: This week’s developments signal mounting stress on the scientific and governance systems that underpin vaccine confidence and access and risk fragmenting systems that were grounded in evidence and transparency. At a moment when routine immunization rates remain fragile and misinformation continues to spread, sustained regulatory clarity, transparent scientific review, and stable engagement in multilateral vaccine processes are essential to maintaining public trust and global health security.
READ:
- F.D.A. Refuses to Review Moderna Flu Vaccine—New York Times
- Vinay Prasad’s Vaccine Kill Shot—Wall Street Journal
- Prasad overruled FDA staff to reject Moderna’s flu vaccine application—STAT
- AMA launching its own vaccine safety, effectiveness review system—The Hill
- Study Finds Declining Perceptions of Safety of Covid-19, Flu, and MMR Vaccines—Annenberg Public Policy Center of the University of Pennsylvania
- Mexico state steps up health screening in schools as measles cases grow nationwide—Associated Press
- FDA’s rejection of Moderna threatens to stifle broader vaccine industry—STAT
- US participating in influenza vaccine meeting: WHO—The Hill
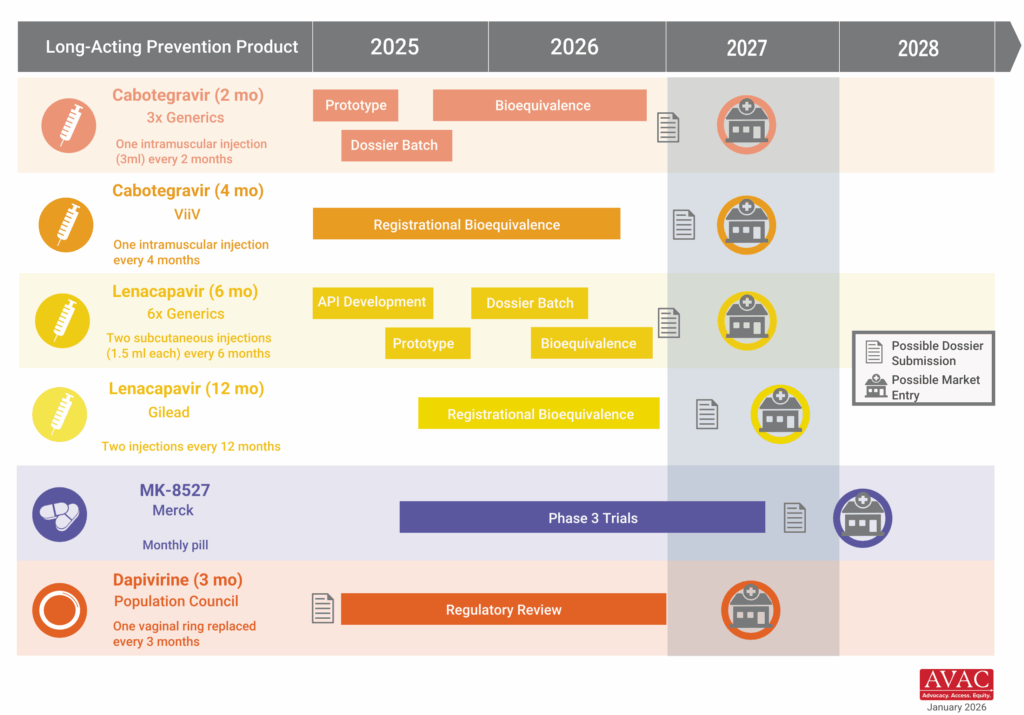
Advocates Urge Early Access Planning for MK-8527
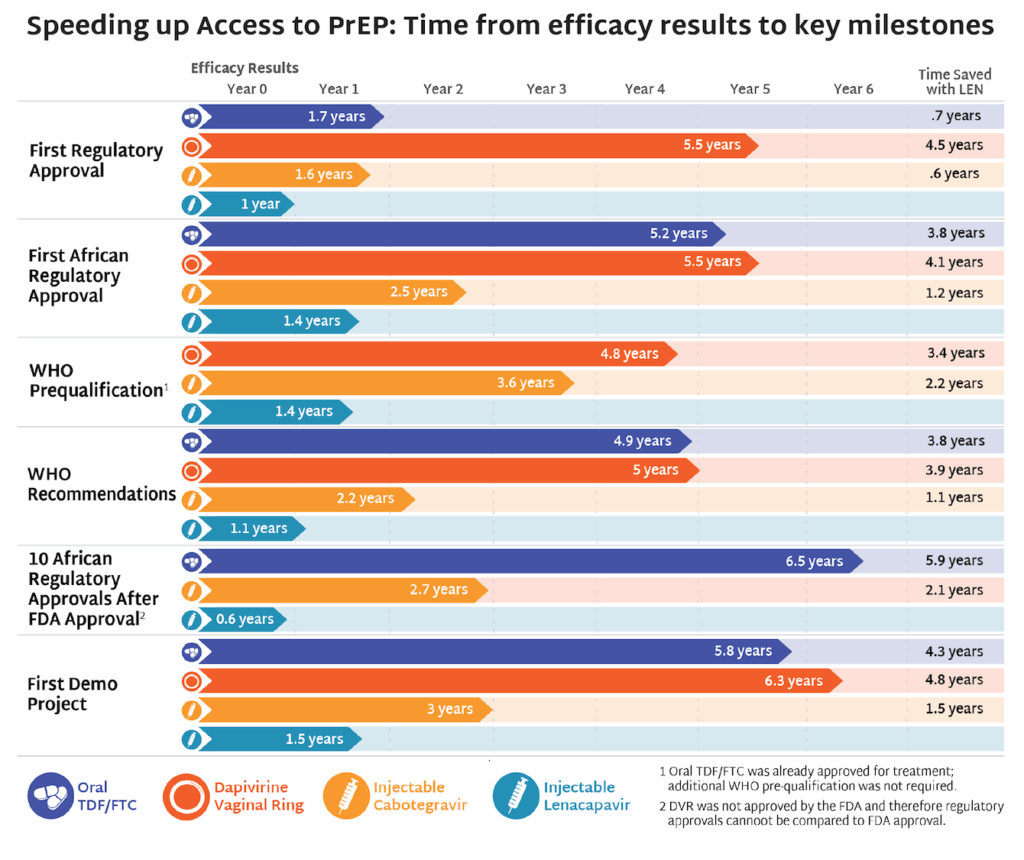
More than 170 groups representing people living with HIV, advocates and providers across 30 countries called on Merck to commit now to a global access strategy for its investigational monthly oral PrEP candidate, MK-8527. Signatories argue that early decisions on pricing, licensing, and regulatory pathways will determine whether the product becomes a transformative addition to the HIV prevention toolkit or follows the slow, inequitable rollout patterns seen with the rollout of oral PrEP. The groups urged Merck to build on the momentum of Gilead’s accelerated work on lenacapavir and pursue regulatory pathways through the European Medicines Agency and the World Health Organization to accelerate approvals in low- and middle-income countries; seek registration in at least 10 high-burden African countries within six months of a major regulatory approval; commit to pricing at or near generic oral PrEP levels (under $40 per person per year); and negotiate non-exclusive voluntary licenses before approval to enable timely generic production.
IMPLICATIONS: This moment reinforces a lesson the HIV field has learned repeatedly: that access needs to be embedded in the design and development of new products. Decisions made during product development around pricing, licensing, regulatory strategy, and manufacturing shape whether innovation translates into impact or inequity. We are already seeing how early, proactive access planning with lenacapavir for PrEP can accelerate timelines, align donors and regulators, and drive what is now the fastest rollout of a new prevention product since oral PrEP. Advocates are pressing Merck to apply those same principles to MK-8527 now, before trials have results, to avoid the delays and disparities that have historically limited uptake of new prevention options in low- and middle-income countries.
READ:
- Merck is urged by patient groups to ensure widespread access to an HIV prevention pill being tested—STAT
- Letter: Ensuring Equitable Global Access to MK-8527 for HIV Prevention
- Speeding Up Access to PrEP: Time from efficacy results to key milestones—AVAC
- The Gears of Lenacapavir for PrEP Rollout—AVAC
AVAC vs. Department of State: One Year Later
This week marks one year since AVAC sued the US government over the sweeping foreign aid freeze that halted HIV and global health programs overnight.
What We’re Reading
- Four States Sue Administration Over Loss of Public Health Funds—New York Times
- EU To Pledge ‚700 Million To Global Fund, Less Than Previous Years—Health Policy Watch
- Will Africa’s health care reset leave patients footing the bill?—Devex
- As The Aid Model Collapses, Africa Is Rewriting Its Health Future Through The ‘African Leadership Meeting’—Health Policy Watch
- Dozens of researchers will move to France from US following high-profile bid to lure talent—Nature
- Malawi struggles to fill development gaps after US aid cuts—Devex
- One year after USAID’s shutdown, Ethiopian aid workers are still struggling—Devex
- UN presses for clarity on nearly $4 billion owed by US as financial crisis looms—France 24
- Zimbabwe’s youth pay the price of US funding drawdown—Devex
- WHO director-general calls plans for US-funded vaccine trial ‘unethical’—STAT
- Kenya second-largest Africa vaccine trial hub—The Star (Kenya)
- By Slashing Foreign Aid, Trump is Fueling the Spread of HIV in Uganda—The Intercept
- UN presses for clarity on nearly $4 billion owed by US as financial crisis looms—France 24
- US Global Health Spending Watch—PIH