This issue covers the Appeals Court opinion in AVAC v Department of State, a violent attack on the CDC fueled by vaccine disinformation, the continued dismantling of the NIH with a potential pendulum swing toward implementation science and away from basic science and clinical research, and a new executive order politicizing federal grants.
AVAC v Department of State Case – Decision Signals Grave Risks
The US Court of Appeals for the D.C. Circuit issued a 2–1 decision in favor of the Administration’s appeal in the AVAC v. US Department of State and Global Health Council v. Trump cases, which challenge the administration’s abrupt and unilateral freeze on foreign aid. The three-judge panel of the Circuit Court of Appeals overturned the lower court’s preliminary injunction, which ordered the Administration to release appropriated foreign aid funds. It didn’t rule specifically on whether the cuts to foreign assistance are constitutional, but instead ruled that the plaintiffs are not allowed to challenge the President’s freeze on Congressionally approved global health funding. In a statement, AVAC’s Mitchell Warren said, “The ruling hands the administration another victory in their intentional effort to destroy decades of progress in global development, diplomacy, public health and human rights. Time and again, this administration has shown their disdain for foreign assistance and a disregard for people’s lives in the United States and around the world. This decision, which we will appeal to the extent possible, further erodes Congress’s role and responsibility as an equal branch of government, and the majority opinion makes the court complicit.”
IMPLICATIONS: This decision signals grave risks for global health and development and the larger role of Congress to maintain it’s Constitutional “power of the purse”. By allowing the executive branch to withhold Congressionally appropriated funds, the court’s ruling threatens millions of lives and sets a precedent for politicizing health assistance.
READ:
- AVAC Condemns Appeals Court Reversal of Order Directing Trump Administration to Spend Foreign Assistance Funds—AVAC statement
- Trump Administration Can Withhold Billions in Aid, Appeals Court Rules—New York Times
- President Trump can continue to withhold billions in foreign aid, court rules—NPR
- US appeals court backs Trump in fight over foreign aid freeze—Devex
- Appeals court says Trump officials can withhold billions in foreign aid—Washington Post
- Appeals Court Reverses Order Directing Trump Administration to Spend Foreign Assistance Funds—Public Citizen statement
Shooting at the US Centers for Disease Control and Prevention
On Friday, a gunman fired shots at the Centers for Disease Control and Prevention (CDC) headquarters in Atlanta, killing one responding police officer. Investigations revealed the shooter blamed the COVID-19 vaccine for his mental health struggles and had anti-vaccine writings in his home. No CDC staff were physically harmed, but the mental and emotional toll is significant.
IMPLICATIONS: This shooting highlights the dangerous convergence of vaccine (and general health) disinformation and targeted violence. The erosion of trust fueled by anti-vaccine rhetoric, especially from political leaders in the Administration, directly endangers the people and programs working to protect communities.
READ:
- Suspect and Officer Are Dead After Shooting Outside C.D.C. in Atlanta—New York Times
- CDC shooter thought COVID vaccine made him suicidal, his father tells police—AP News
NIH Shift to Implementation Science and Further Takedown of the NIH
The dismantling of US support for global health R&D continues to accelerate. As Science magazine reported this week, the NIH is considering a dramatic shift in its HIV research budget away from basic science toward implementation science, with an emphasis on how best to use existing tools (including lenacapavir for PrEP) to end the epidemic in the US. This news follows the defunding of mRNA vaccine development under the Biomedical Advanced Research and Development Authority (BARDA) earlier this month and a dangerous overhauling of long-standing peer review processes for NIH grants (see more below). AVAC’s updated tracker of cuts to HIV R&D includes these latest developments.
IMPLICATIONS: LEN for PrEP could have a critical role to play in ending the epidemic in the US and around the world, and implementation science is definitely needed to optimize its potential impact. But LEN is not the only intervention needed, and it will be critically important to embed any implementation science agenda into a comprehensive “R&D and delivery” research agenda that shows the appropriate balance of the overall HIV/AIDS research portfolio. The proposed shift comes at a particularly challenging time, with the President’s fiscal year 26 (FY26) budget request proposing an additional 40% cut to NIH. The field faces a tipping point—if the field doesn’t act now to define itself, the new Administration will. Advocates are pushing back with legal action, Congressional engagement, and an urgent focus on the People’s Research Agenda, a framework to ensure research is driven by communities and focused on equitable access and real-world impact.
READ:
- NIH ponders overhauling HIV budget to capitalize on prevention breakthrough—Science
- HIV Prevention R&D at Risk—AVAC
- RFK Jr has slashed vaccine research. You need to know how perilous that is for the world—The Guardian
New Executive Order Gives Political Appointees Control Over Federal Grants
The US President signed an executive order granting political appointees unprecedented control over federal research grants. This allows them to review funding decisions, terminate existing grants at will, and delay new solicitations. Historically, grants have been allocated through peer-reviewed processes, so this policy shift threatens to further entrench partisan political priorities in scientific funding.
IMPLICATIONS: This new level of political control over research funding poses serious risks to HIV and broader global health R&D. Agencies are continuing to lose the ability to rely on predictable, science-driven funding streams. The new executive order severely disrupts decades of equitable and evidence-based research support.
READ:
- Trump signs order giving political appointees oversight of federal grants—The Hill
- Trump executive order seeks to centralize control of grantmaking under political appointees—STAT
- Donald Trump Announces Major Change to ‘Frivolous Grants’—Newsweek
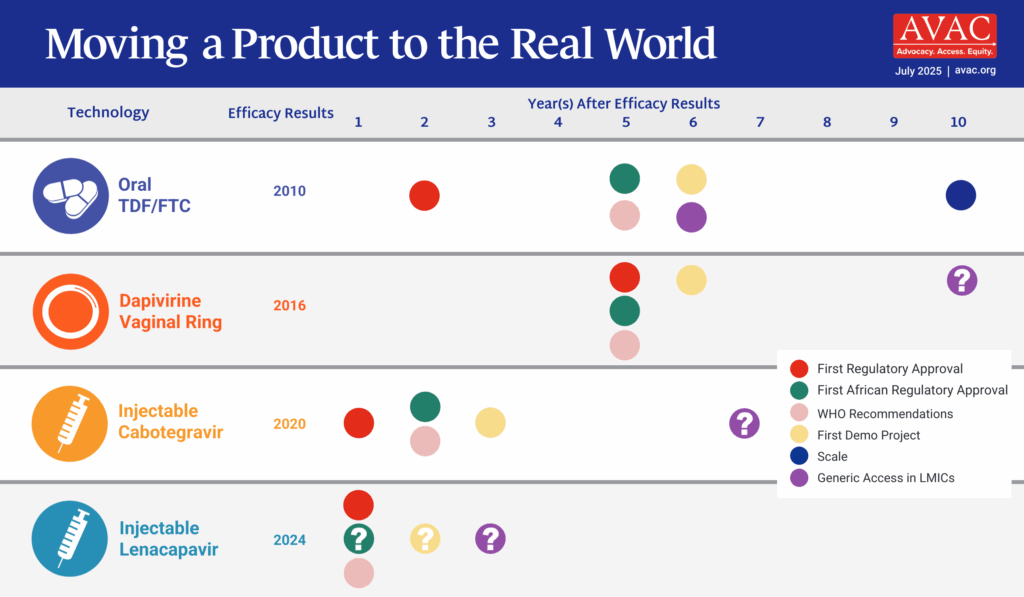
Getting PrEP Rollout Right This Time: Considerations for LEN for PrEP Introduction
This new resource shares insights and recommendations for the introduction of lenacapavir for PrEP building on lessons from over a decade of PrEP implementation.
What We’re Reading
- No One in the White House Knows How to Stop Ebola—The Atlantic
- Elton John AIDS Foundation plugging gaps in HIV funding—The Lancet
- WATCH: A Conversation on the Future of PEPFAR With One of Its Chief Architects—Tom Frieden Substack with Mark Dybul
- Is PEPFAR’s Data Going Dark?—To End a Plague Substack
- If We Want to Save Public Health, We Need to Get Out of Our Bubble—The Nation
- Exclusive: USAID officials tour missions worldwide as agency shutter—Devex
- How HIV funding cuts are undermining years of progress in Zimbabwe—Doctors Without Borders
- Inside SA’s multi-million rand plan to fill US funding void—Spotlight
- ‘Kennedy’s case against mRNA vaccines collapses under his own evidence—STAT
- Trump administration threatens to strip Harvard University of lucrative patents—The Guardian
- Vinay Prasad returns to the FDA, weeks after his ouster—STAT
- HHS revives child vaccine safety panel sought by anti-vaccine activists—Washington Post
- Breaking barriers, changing paradigms: Africa’s radical agenda for HIV sustainability—Frontiers in Reproductive Health
- ‘A whole body of health-equity research is being disappeared’ — why I resigned from the NIH—Nature
Updated Resources
- PEPFAR Program Status, amfAR
- Save PEPFAR, Save Lives: Rally & Civil Disobedience in DC, Housing Works
- Addressing Transgender Erasure in HIV Clinical Trials: The Scorecard for Transgender and Gender-Diverse Inclusion, American Public Health Association
- Long-Acting PrEP Market Assessment for Key Populations, AVAC, GBGMC
- The Global Need for Long Acting PrEP Among Key Populations: Forecasts of Global Demand 2025-2030, AVAC, Avenir Health, GBGMC