This week the Global Fund and Gilead announced next steps in the process to rollout injectable lenacapavir, the new UNAIDS Global AIDS Update was released, and South Africa saw a partial reprieve for NIH-funded research. We also track AVAC’s court case against the foreign aid freeze and Congressional advocacy to protect NIH funding. Read on, and be sure to follow AVAC next week as we cover the important discussions at the International AIDS Society (IAS) 2025 conference.
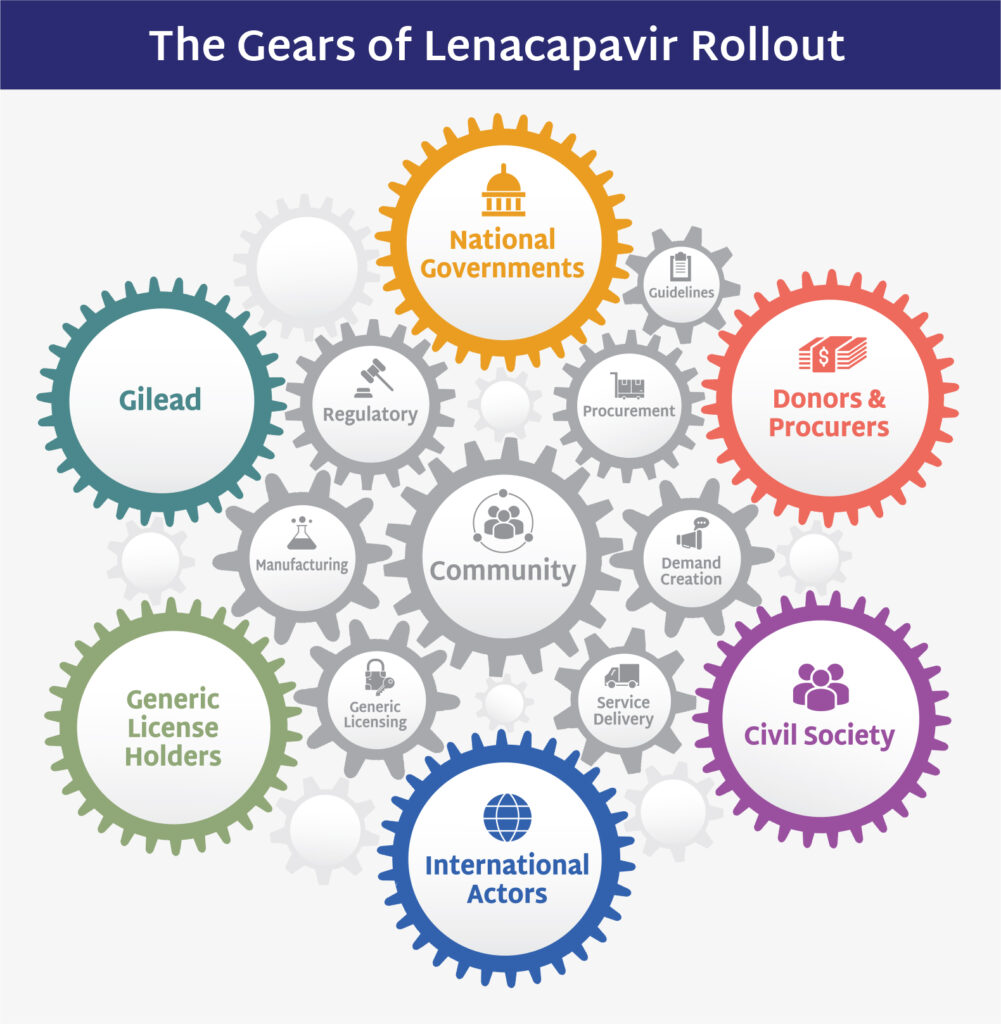
The Global Fund and Gilead Announce Next Steps on LEN for PrEP
The Global Fund and Gilead Sciences announced an access agreement to procure injectable lenacapavir (LEN) for PrEP, an important step in the process of rolling out LEN. The announcement re-confirms their ambition from December with PEPFAR to reach 2 million people over three years with LEN, once WHO recommendations are in place, which are expected to be announced on Monday at the IAS conference.
IMPLICATIONS: While these announcements mark welcome progress in advancing LEN rollout, key questions remain. The announcements did not include specific volumes or price – and the target of 2 million people over three years is stated as an ambition, not a commitment to procure the full volume required to meet this target. In addition, achieving this ambition will depend on Global Fund replenishment for the next three-year budget. As AVAC and partners have noted, ambitious targets require coordinated, transparent planning and financing to prevent delays and ensure LEN fulfills its promise. Check out AVAC’s new brief that explains it all and proposes an even more ambitious introduction.
READ:
- Global Fund Secures Access to Breakthrough HIV Prevention Drug Lenacapavir for Low- and Middle-Income Countries—Global Fund statement
- Gilead Imposes Price Secrecy on Global Fund Over Breakthrough HIV Prevention Shot, Blocking Transparency and Accountability—Health Gap and partners
- Gilead to provide HIV prevention drug to 2 million people in lower-income countries at cost—STAT
- Gilead, Global Fund finalize plan to supply HIV prevention drug to poor countries—Reuters
- Now What with Injectable LEN for PrEP: How to Translate Ambition into Accelerated Delivery and Impact—AVAC
UNAIDS Releases Global AIDS Update 2025
Ahead of next week’s IAS 2025 Conference, UNAIDS released its Global AIDS Update 2025, AIDS, Crisis and the Power to Transform, showing that another 1.3 million people acquired HIV in 2024, which is far from the target of reducing infections below 370,000, by 2025. The report warns of severe disruptions to HIV prevention services as US foreign aid and global health financing abruptly collapsed. “This is not just a funding gap—it’s a ticking time bomb,” Winnie Byanyima, UNAIDS executive director said at the launch. She emphasized that community-led services, which are critical for reaching marginalized populations, are being defunded at alarming rates.
IMPLICATIONS: This year’s report underscores the need for bold, sustained action and funding, calling on donors, governments, and communities to step up and invest urgently in scaling proven prevention, including PrEP in all its forms, to protect gains and advance the goal of ending AIDS as a public health threat by 2030.
READ:
- Countries must urgently step up to transform their HIV responses amid an international funding crisis that risks millions of lives—UNAIDS statement
- Millions at Risk of HIV Infection and Death After US Funding Cuts, Warns UNAIDS—Health Policy Watch
- Poor nations try to bridge AIDS funding gap but prevention efforts dwindle, UN says—Reuters
- IAS 2025, the 13th IAS Conference on HIV Science—AVAC, including links to our Roadmap to find sessions where prevention and the larger issues of global health equity and sustainability are in the spotlight. You can download it as a sortable spreadsheet or PDF.
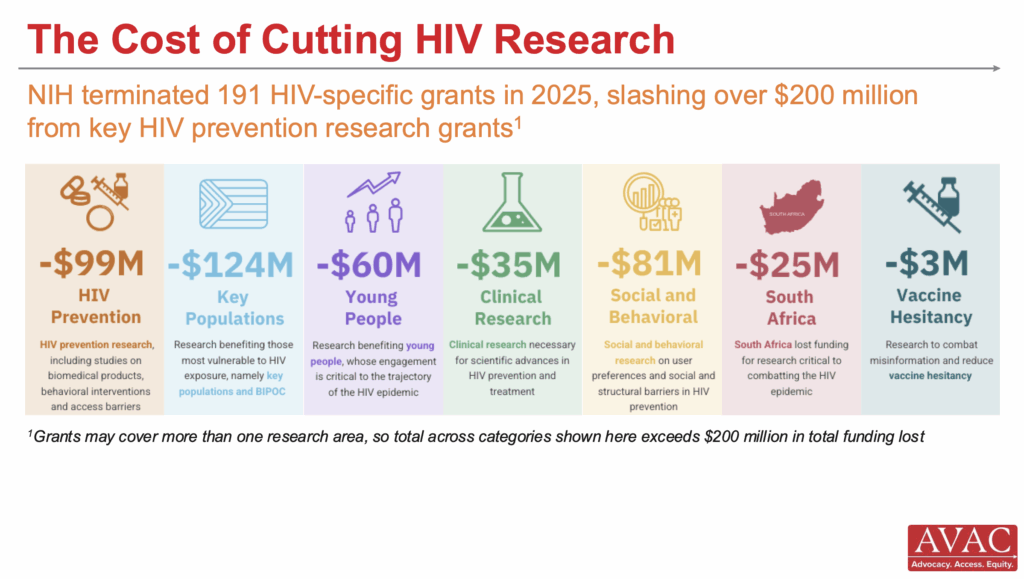
NIH Lessens Blow to South Africa Research
The National Institutes of Health (NIH) shared guidance with its staff on an “alternative payment scheme” that could allow human clinical research studies in South Africa to continue as “supplements” to existing grants until the agency puts a new tracking system in place, which is expected September 30. While new grant awards to South Africa are still blocked, “ongoing prime awards to South African researchers, ‘may proceed’,” Science reports. The NIH also lifted a hold on many payments for existing grants to South Africa.
IMPLICATIONS: This slight reprieve for South African research is a positive step forward, but damage has already been done through months of staff layoffs, paused trials and stalled collaborations. And the uncertainty surrounding NIH’s broader, ideologically driven crackdown on grants continues to impact science, collaboration and progress.
READ:
- NIH restores grants to South Africa scientists, adds funding option for other halted foreign projects—Science
- Democrats stage a science fair of canceled grants to show what’s been lost—Science
- NIH-funded Human Trials Outside US Get Temporary Funding Reprieve—Health Policy Watch
AVAC vs. Department of State in the Foreign Aid Freeze
Oral arguments in the AVAC vs. US Department of State lawsuit (joined with Global Health Council vs. Trump) were heard in the Washington, DC Circuit Court of Appeals on Tuesday. The cases seek emergency relief from an Executive Order that inhumanely froze all funding for foreign assistance and challenge the Administration’s shutdown of USAID and foreign aid. AVAC and GHC, and their partner have consistently won in the District Court, and the government appealed the judgements against them to this higher court. A panel of three Circuit Court judges heard the arguments and are expected to rule on the case by August 15. The Administration’s defense argued that the Congressional appropriations are merely “ceilings” rather than binding requirements. The panel of judges seemed to push back, noting that US law and constitutional separation of powers give Congress authority to set spending levels, not the executive branch.
IMPLICATIONS: The hearing exposed the Administration’s strained arguments attempting to justify the foreign aid freeze and underscored the high stakes of the case: whether the Administration can disregard congressionally mandated funding for global health and foreign assistance programs, including PEPFAR and USAID’s initiatives. Watch AVAC’s Mitchell Warren on the latest in the case.
READ:
- Audio recording of the hearing
- To justify aid cutoff, Trump Administration lawyer uses bizarre logic.—David Bryden of Partners in Health on LinkedIn
HIV Organizations and Advocates Host NIH Congressional Briefing
Scientists and leaders in infectious disease research presented at a Congressional briefing, co-hosted by AIDS Action Baltimore, Treatment Action Group, AVAC and 11 other organizations, on the lifesaving impact of NIH-funded infectious disease research. They shared the impact of NIH investment—calling the NIH a “national treasure”—which has driven innovations in HIV prevention, cure, and treatment, TB diagnostics, viral hepatitis treatment, and pandemic preparedness, and underscored how recent funding cuts and threats to NIH-supported research jeopardize public health progress. They urged Congress to sustain support for lifesaving NIH research.
IMPLICATIONS: The briefing reinforced that continued and expanded NIH investment is critical not only for advancing science, but also for equitable global health outcomes, pandemic readiness, and US health security. As Congress debates the Fiscal Year 2026 budgets and potential rescissions for past years, advocates called on policymakers to protect NIH funding, reject proposed cuts, and recognize research as essential infrastructure for global and domestic health. READ:
- 13 Renowned HIV Organizations Unite to Host Congressional Briefing Advocating for Restored NIH Funding—Presswire
- Lifesaving NIH Infectious Disease Research, NIH Congressional Briefing document
- Reject the Rescissions. Defend Global Health—Global Health Council
IAS 2025: What you need to know
Follow AVAC’s roadmap to find sessions where prevention and the larger issues of global health equity and sustainability are in the spotlight.
What We’re Reading
- We must fill the void in global HIV care without PEPFAR—BMJ
- Kennedy abruptly cancels preventative care committee meeting—STAT
- NIH director is replacing his top outside advisory board—Science
- When an HIV Scientific Breakthrough Isn’t Enough—Bloomberg
- You Don’t Have to Be a Doctor to Understand This—New York Times
- Health aid saves lives. Don’t cut it.—Bill Gates, LinkedIn
- Planned Parenthood sues Trump administration over planned ‘defunding’—Reuters
- NIH budget cuts threaten the future of biomedical research — and the young scientists behind it—LA Times
- ‘The biomedical research enterprise is under attack’—WBUR On Point with Tony Fauci
- Why Calling RFK Jr. ‘Anti-Science’ Misses the Point Battling over truth, facts, and evidence doesn’t work in a post-expertise world—NY Magazine
- Meet The Examination, a nonprofit watchdog for global health—Poynter
- Cambodia to combat HIV with revolutionary vaginal ring—Khmer Times
Updated Resources
- Donor Government Funding for HIV in Low- and Middle-Income Countries in 2024, KFF
- Principles of a responsible transition of American leadership to end AIDS: Strategic transition or pandemic resurgence?, Friends of the Global Fight Against AIDS, Tuberculosis and Malaria
- Global Fund Replenishment 8 Scenarios, DataEtc.
- Science & Community Impacts Mapping Project (SCIMaP), SCIMaP
- Now What with Injectable LEN for PrEP: How to Translate Ambition into Accelerated Delivery and Impact, AVAC
- The Scientific Journey of Lenacapavir, AVAC
- LEN Generics — Can we go faster?, AVAC
- Research Matters, AVAC, HIVMA, TAG