Looking backward and then into the future, this chart shows actual HIV rates alongside projected rates with and without current prevention strategies (PrEP, VMMC, and free condoms).
HIV Incidence, Age 15-49
Global PrEP Uptake and PEPFAR’s Role, 2016-2024
AVAC’s Global PrEP Tracker has documented cumulative PrEP initiations on a quarterly basis for nearly a decade. This graph presents the final data collected while PEPFAR was fully operational—PEPFAR support was responsible for 79% of PrEP uptake globally in the last year and reached 83% by the end of September of 2024. Data on the fourth quarter of 2024 is inaccessible since PEPFAR was taken offline in late January.
PxWire Volume 15, Issue No. 1
In this special edition of Px Wire, AVAC is going beyond a quarterly update of biomedical HIV prevention. In this issue, we look at how the new US Administration’s attack on global health can be expected to devastate HIV prevention, including the capacity to deliver existing PrEP options, the scale up of new PrEP products, and the paralyzing impact on research and development. A PDF version of this report is also available.
From Research to Rollout: The impact of USG global health pullout


The United States’ presidential regime has launched a sustained, multi-pronged attack against foreign assistance, scientific inquiry, due process and good governance. It threatens economies, human rights, international partnerships, global health at large, and the rule of law. For HIV prevention, a single sentence, issued in a February 6 advisory from the US Department of State, has derailed the entire field, potentially setting back the HIV response by years, if not decades. Read on for resources to support your advocacy and fortify our solidarity at this critical time.
Progress in PrEP Uptake: Threatened
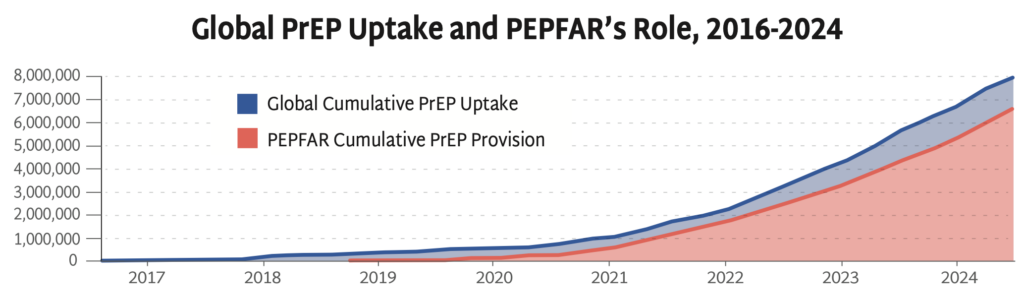
PEPFAR has been pivotal to accelerating PrEP uptake, significantly expanding HIV prevention coverage. The freeze on foreign aid prohibits funding to PEPFAR’s PrEP programs and poses a serious threat to global efforts to control the epidemic.
AVAC’s Global PrEP Tracker has documented cumulative PrEP initiations on a quarterly basis for nearly a decade. This graph presents the final data collected while PEPFAR was fully operational—PEPFAR support was responsible for 79% of PrEP uptake globally in the last year and reached 83% by the end of September of 2024. Data on the fourth quarter of 2024 is inaccessible since PEPFAR was taken offline in late January.
At the time of the foreign aid freeze, PrEP uptake had reached 8 million initiations since 2016, an achievement that’s taken almost 10 years to reach—too slow and too small to reach UNAIDS targets, but a robust foundation to finally accelerate PrEP uptake with next-generation PrEP. Current US policies, instead of expanding PrEP coverage, are leading to the closure of programs, and will reverse global progress against HIV.
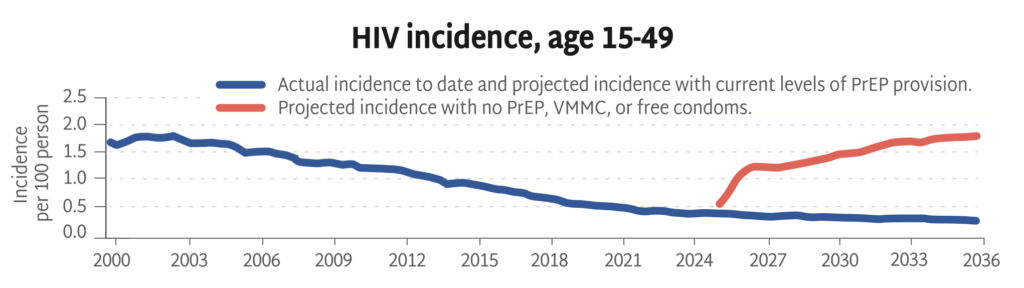
Without primary prevention, the HIV epidemic is poised to rage on, with incidence among adults on track to triple over the next ten years. This HIV Synthesis model, developed by the HIV Modelling Consortium, estimates the impact of stopping all HIV prevention services across Africa from now through 2036—including PrEP, voluntary medical male circumcision (VMMC), and free condom distribution.

For the last 8 years, AVAC has proudly worked with PEPFAR to document PrEP uptake and its impact around the world. That stopped in January with a stop work order from the US government. But protecting access to PrEP is vital. Are you leading a PrEP program? Whether supported by PEPFAR or not, we invite you to work with us to ensure global data on PrEP is not lost. Find us at [email protected].
PrEParing for New Products: Is rollout still possible?

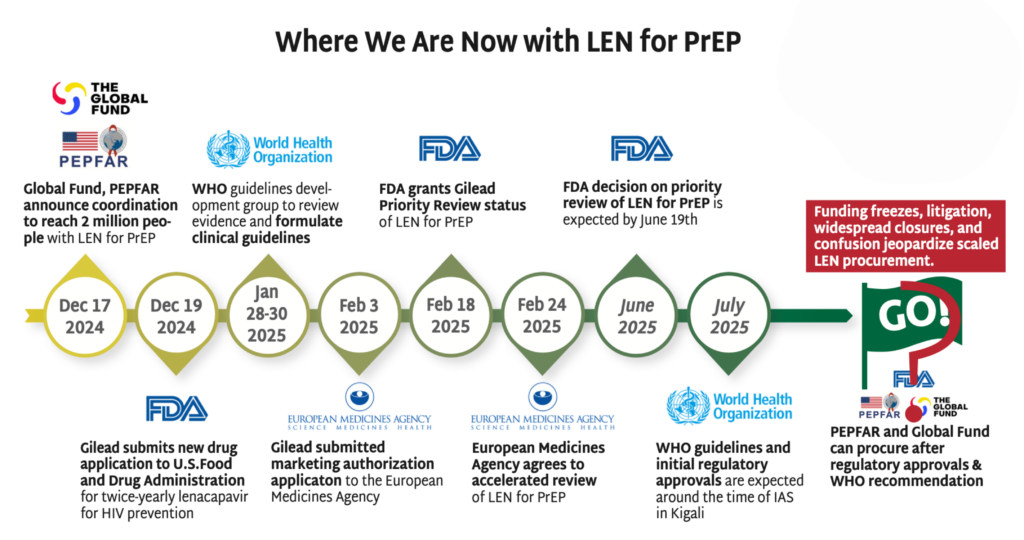
Read more in The Gears of Lenacapavir for PrEP Rollout.
The chaos in foreign assistance programs (including discontinuation of major PrEP programs), cuts in staffing and new demands on donor commitments will make decisions on the procurement of LEN for PrEP more complex and uncertain. In December 2024, the Global Fund and PEPFAR announced a plan to reach 2 million people with LEN for PrEP over three years. Exactly how funding to support this unprecedented introduction program will move forward, in the absence of significant US investment, is far from certain. The other stakeholders, including Global Fund, Gilead, CIFF and the Gates Foundation expressed commitments to the deal, but major questions remain. In the meantime:
- Gilead’s production of LEN for PrEP is continuing, as is the technology transfer to generic license holders.
- The FDA granted Gilead priority review status for LEN for PrEP, with a decision due by June 19, 2025.
- The EMA has agreed to an accelerated review of LEN for PrEP for both European access and as part of the EU-Medicines for all (EU-M4all) program, reducing the review from seven months to five months.
- The WHO’s Guideline Development Group (GDG) met in January, and WHO is expected to issue guidelines by July 2025.
The Latest R&D in the Prevention Pipeline: Supported or undermined?

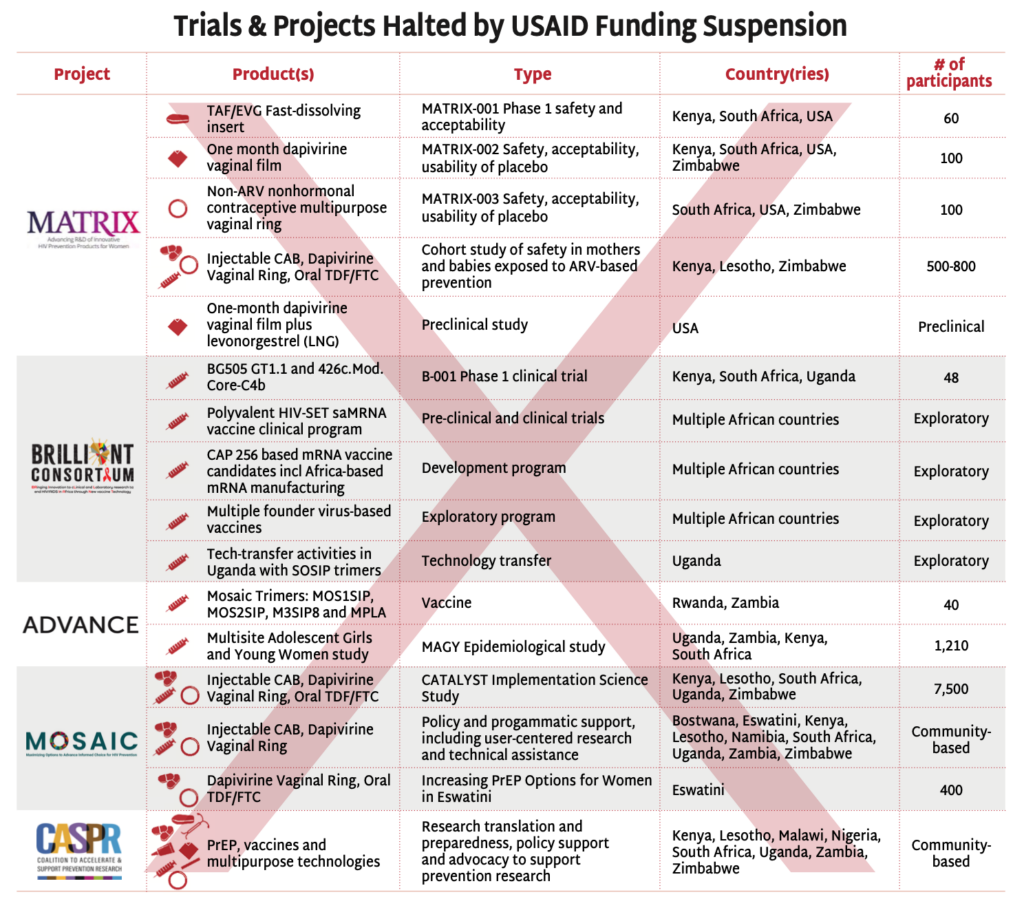
The stop-work orders have disrupted USAID-supported HIV prevention research, halting critical investigations in vaccine and next-generation PrEP strategies.
- The BRILLIANT and ADVANCE projects’ clinical, preclinical, and experimental trials testing HIV vaccine candidates have been suspended.
- The MATRIX projects’ driving innovation with next-generation PrEP and MPT products, fast-dissolving inserts and vaginal films and rings, have been forced to stop their clinical trials.
- The MOSAIC projects’ have suspended all implementation science activities, including the CATALYST study, investigating choice among oral PrEP, injectable cabotegravir and the dapivirine vaginal ring. Other implementation studies are continuing, but access to the commodities, much of which was procured by PEPFAR is questionable. See AVAC’s Integrated Study Dashboard for details.
- The Coalition to Accelerate and Support Prevention Research (CASPR) has also been paused. Led by AVAC in partnership with a number of leading African civil society organization, CASPR focuses on building an enabling environment for HIV prevention R&D. (Note: In early February, AVAC lead a lawsuit against the State Department seeking emergency relief from the freeze on foreign assistance, including funding for CASPR. The case, AVAC v. United States Department of State, is pending.)
These disruptions delay the development of urgently needed HIV interventions and threaten the sustainability of research infrastructure all over the world, with particularly egregious impacts on the research capacity of regions most impacted by the epidemic.
The abrupt suspension of these trials also raises serious ethical concerns. Stopping trials mid-course undermines trust in research, jeopardizes community engagement, and abandons participants who volunteer their bodies for scientific discovery. It will take years to build back this critical infrastructure—for HIV research and beyond—as well as the community partnership and trust needed to ensure smooth and ethical research.
Prevention Playlist
AVAC develops a wide range of resources to inform decision making and action. Check out the latest:
Join
- Subscribe to Global Health Watch: AVAC’s weekly newsletter dedicated to breaking down critical developments in US policies and their impact on global health. avac.org/signup
- Seeking Visuals and Videos: Leading groups in Washington, DC, are urgently trying to collect videos and photos documenting the impact of the US government’s foreign aid freeze, such as clinic closures despite the waiver. Non-professional phone videos and photos are welcome. Send to [email protected] for more details
- CHANGE: In response to the unfolding crisis, more than 1,300 people from civil society organizations around the world have launched CHANGE—Community Health & HIV Advocate Navigating Global Emergencies—a coalition formed to support urgent action. [email protected]
Use
- Graphic of studies of injectable cabotegravir and the dapivirine vaginal ring in eastern and southern Africa, AVAC
- Most Lifesaving Services Remain Paused: A Rapid Assessment of the PEPFAR Stop Work Order, amfAR, CHANGE, Data ETC
- What Effect are HIV Programmes Having in Africa, The HIV Modelling Consortium
- PEPFAR & Global Fund Support for HIV Programs, amfAR & Data ETC
Watch/Listen
- [LISTEN] Would PEPFAR Survive Trump—and what would it look like?
- The Impact and Implications of Recent US Government Federal Funding Reductions on Health Programmes, The Steve Biko Centre for Bioethics at The University of the Witwatersrand event recording
Read
- AVAC v United States Department of State. On February 10, 2025, AVAC and another nonprofit organization sued the new US Administration, seeking emergency relief from an Executive Order that inhumanely froze all funding for foreign assistance, AVAC
FAPP Response to NIH Cuts to Indirect Research Costs
Federal AIDS Policy Partnership Research Working Group urges Congressional leaders to support the restraining order preventing the National Institutes of Health (NIH) from unlawfully stripping funds that sustain cutting-edge medical and public health research at universities and research institutions nationwide. Read the full letter.
Community-Led Monitoring: Transforming the HIV response in Malawi

For several years now, Community-Led Monitoring has been on the rise in the HIV response, and particularly in East and Southern Africa. Known as CLM for short, it’s a tactic being championed and implemented to ensure that communities play a direct role in monitoring and improving HIV services.
In this episode of PxPulse: The Advocacy Chronicles we delve into CLM in Malawi, where civil society and communities are successfully using this approach to connect government decision makers to the gaps in HIV services and to what people really need. Thanks to persistent advocacy, both PEPFAR and Global Fund now recognize, through their funding, the critical role of CLM.
The episode features David Kamkwamba, a journalist and health advocate and the former chair of the Civil Society Advocacy Forum on HIV and related diseases, commonly known as CSAF tells us what advocates have accomplished in Malawi and just how they did it. CSAF and AVAC are partners in the Coalition to Build Momentum, Power, Activism, Strategy & Solidarity (COMPASS) which has supported extensive work on community-led monitoring in Malawi and across the region.

Listen
Download the full podcast (19:55) or listen below.
Resources
Gears of Lenacapavir for PrEP Rollout
The Gears of Lenacapavir for PrEP Rollout outlines a transformative opportunity in the global fight against HIV, coming at a pivotal moment when the scale-up of PrEP has shown remarkable progress but remains insufficient to achieve a transformational impact on HIV incidence and the trajectory of the epidemic. This effort demands a coordinated response from governments, donors, civil society, and the private sector to ensure rapid implementation, equitable access, and sustainable impact. By leveraging lessons from previous PrEP interventions, aligning financing mechanisms, and prioritizing underrepresented regions, stakeholders can overcome systemic barriers and maximize the public health potential of this innovative long-acting prevention tool.
With anticipated regulatory approvals and production scaling, the plan targets over 2.5 million LEN users in low- and middle-income countries by 2027. With a focus on addressing structural barriers like stigma, healthcare inequities, and restrictive policies, alongside integrating generics into national programs, the roadmap aims to build on existing progress while accelerating the pace of HIV prevention.
The Gears Framework for Lenacapavir for PrEP Rollout
The gears framework for lenacapavir scale-up brings together a coalition of essential stakeholders, each contributing to the successful, sustainable integration of this HIV prevention tool into global health systems.
Excerpted from the Gears of Lenacapavir for PrEP Rollout report.
Clinical Trial Designs in HIV Prevention
An introductory slide deck covering the basics of trial design in product development — definitions, phases, randomized control trials, and more.
An Advocacy Chronicle on Universal Healthcare in Tanzania, with Atuswege Mwangomale of Sikika

In this episode of PxPulse: The Advocacy Chonicles, Atuswege Mwangomale goes deep on the advocacy work behind the passage of Tanzania’s Universal Healthcare Law. Atu serves as Head of Health Programs for Sikika, a Tanzania-based advocacy organization with a long track record of promoting best practices in governmental financing in the health sector, and advocating for improved health outcomes. Sikika, along with AVAC, is also a member of the COMPASS coalition, which uses data and coalitions across Africa to identify strategic campaigns to advance the HIV response.
Sikika’s advocacy has been crucial to the ultimate passage of Tanzania’s Universal Health Insurance Bill in 2023, but full funding must still be secured for the law to achieve full impact.
Atu explains the promise of UHC in Tanzania, how Sikika won the trust of government allies, and why working in coalition was essential to success.
Listen
Resources
- Universal Health Insurance: Transforming Tanzania’s Healthcare System, Diplomatist
- Tanzania enacts the Universal Health Insurance Bill, P4H
- Executive Summary: The State of Universal Health Coverage in Africa – Africa Health Agenda International Conference, AHAIC
- Advancing Universal Health Coverage in Tanzania, Sikika
People’s Research Agenda (2024)
The 2025 update to the People’s Research Agenda is available here.
Led by AVAC alongside a network of partners, the People’s Research Agenda puts forward recommendations to diversify and strengthen the HIV prevention pipeline, enhance investment and financial support for HIV prevention research and development, and guide an advocacy strategy that truly addresses the needs of communities across the prevention pipeline.
The PRA is a living document developed through intentional consultative processes that used multiple modalities, including surveys, focus groups, convenings, to gather insights about the processes and products needed to actualize HIV prevention justice.
In this summary of the People’s Research Agenda, you’ll find the PRA’s core insights into the processes involved in HIV prevention research and implementation, and the types of products that should be developed through these processes.
