This graphic shows currently available options for HIV prevention, newly approved and recommended treatment, and those in development.
The HIV Prevention Pipeline
Long-Acting PrEP: Priority Slides
Slides with the current status and proposed priorities for long-acting PrEP products, regulatory approvals, planning & budgeting, delivery, and much more. PDF and PPT versions available above. For slide 1, current status, click here. Slide 2, proposed priorities, is also available.
For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Avac Event
The Fight for Health Justice in an Age of Retreat

Press Release
AVAC Applauds Agreements to Accelerate Market Development for Lenacapavir for PrEP
New York, NY, September 24, 2025 — AVAC welcomes parallel announcements from the Gates Foundation and Unitaid on strategic investments to accelerate the development of, access to and price reduction for generic versions of injectable lenacapavir (LEN), the highly effective six-monthly injection for HIV PrEP.
“These investments are a vitally important step in translating the remarkable science of LEN into public health impact,” said Mitchell Warren, executive director of AVAC. “With these agreements, injectable LEN for PrEP gets closer to the price of daily oral PrEP, $40 per person per year, for low and middle-income national governments. This means national programs in many, but not all, countries can begin planning for 2027, at which time ongoing oral PrEP and LEN use will be available at similar prices, meaning many countries will be truly able to offer people who need prevention choice when it comes to the PrEP method that best meets their needs. This could be a transformational moment in HIV prevention if political will, coordination, and further procurement investment meet this moment to deliver LEN with speed, scale and equity to all communities and populations who need and want prevention options. Many questions remain, but in this current environment, we need to seize opportunities and good news when we can.”
These new commitments to accelerate access to generic versions of LEN come on the heels of the Global Fund and PEPFAR re-committing to their December 2024 announcement of reaching two million people with LEN for PrEP within three years, with drug supplies coming from the originator company, Gilead Sciences. Among the outstanding questions from these new commitments is the price of the required oral loading dose for LEN, which is needed to achieve high efficacy. While the cost of ongoing use of LEN for PrEP would be similar to the cost of a year of daily oral PrEP, anyone initiating or restarting LEN for PrEP needs an oral loading dose of LEN. This oral loading dose is not included in the $40 price mentioned in the Gates and Unitaid deals and will add between $15-$17 in the first year of anyone initiating or re-starting LEN, and supply chains and purchasers need to include this extra cost in their calculations.
“The ‘two million in three years’ ambition from Global Fund and PEPFAR must be seen as a floor and not a ceiling,” said Warren. “The global PrEP data that AVAC tracks show a more ambitious goal, getting LEN to at least 1.5 million next year alone, is achievable and necessary. Ultimately, LEN must reach more than five million people per year to have real impact, build a sustainable market, and drive prices down even further. This means we must act faster and think bigger.”
AVAC calls on all stakeholders to do their part. Next steps require coordinated action and further investment to ensure the creation of a viable and sustained market.
“This is the moment to ensure that LEN for PrEP lives up to its full potential, and to hold each other accountable for what must happen next,” said Wawira Nyagah, AVAC’s director of product introduction & access. “Demand creation and program design for LEN must be fully resourced, evidence-based and community-centered. Volume commitments, manufacturing, and supply chains must be sustained and stable. But to make a difference at a global level, the HIV response must go beyond these essential, but minimum, steps with a bold vision to accelerate the entry of generics and trigger a virtuous cycle of price drops, which further drive-up PrEP use.”
LEN, developed by Gilead Sciences, is a twice-yearly injectable PrEP option that showed nearly complete protection against HIV in the landmark PURPOSE 1 and 2 trials. Science Magazine named LEN the “Breakthrough of the Year” in 2024, a recognition that reflects its enormous potential. But fulfilling this potential is far from certain, and all stakeholders have critical work to do, as detailed in AVAC’s 2024 publication, Gears of Lenacapavir for PrEP Rollout.
AVAC’s publication, Now What with Injectable LEN for PrEP How to Translate Ambition into Accelerated Delivery and Impact, includes forecasts demonstrating that instead of 2 million people in three years, the world could reach at least 1.5 million people in just one year. Gilead has confirmed that they can manufacture enough injectable LEN to reach in excess of 5 million LEN users over the next three years. These numbers suggest what is possible and this is no time to think small.
“To achieve true impact against HIV requires early commitments from additional donors to procure large volumes of LEN, which will enable a bigger rollout, exceeding targets, and reaching more people who need PrEP in more places, which in turn secures the kind of market scale that accelerates further prices reductions,” said Nyagah. “It requires country regulators, ministries of health, implementers, advocates and communities where HIV prevention is needed to prepare with policies and programs that will succeed in connecting people with products that work in the context of their lives. The field has learned these lessons before. Technology alone gets you nowhere; it’s delivering the product with speed, scale and equity that gets the job done.”
###
About AVAC
AVAC is an international non-profit organization that provides an independent voice and leverages global partnerships to accelerate ethical development and equitable delivery of effective HIV prevention options, as part of a comprehensive and integrated pathway to global health equity. Follow AVAC on Bluesky and Instagram. Find more at www.avac.org and www.prepwatch.org.
Avac Event
South African AIDS Conference (SAAIDS)
The 12th SAAIDS Conference 2025, under the theme: Unite for Change – Empower Communities and Redefine Priorities for HIV/AIDS will be held in Johannesburg 8-11 September.
Resources
- Conference website
- Program outline
- Prevention Advocates’ Corner program (forthcoming)
If you’re attending, don’t miss a keynote plenary where AVAC’s Mitchell Warren will share perspectives on the future of HIV prevention, and an important satellite on Wednesday, Bridging the Gap and Identifying Opportunities: Innovative Strategies to Accelerate HIV Prevention, Treatment, and Care for Key and Vulnerable Populations in South Africa.

Press Release
AVAC Condemns Administration’s Further Actions to Dismantle and Deconstruct U.S. Government Vaccine Research and Delivery Infrastructure
Contact: [email protected]
New York, NY, August 6, 2025 — AVAC condemns a series of actions taken by the U.S. presidential administration to dismantle U.S. leadership in research, development and delivery of lifesaving vaccines. The latest move announced yesterday to defund grants issued by the Biomedical Advanced Research Development Authority (BARDA) for research and development of the mRNA vaccine platform is part of a broader pattern to systematically decimate investments in vaccine development programs and delivery systems and continue to sow ideologically driven vaccine misinformation. Without U.S. government leadership, the country and the world will remain woefully unprepared for ongoing and emerging pandemic threats.
“We are disappointed and alarmed to see the government continue an onslaught against vaccine science and confidence,” said Mitchell Warren, Executive Director of AVAC. “Actions to take apart the CDC’s Advisory Committee on Immunization Practices (ACIP), to cancel grants to the Consortia for HIV/AIDS Vaccine Development (CHAVDs), to cease contributions to Gavi, The Vaccine Alliance, and now to cancel BARDA support for mRNA vaccines are a red alert all around the world, signaling the U.S. retreat from advancing vaccine development and delivery.
This week’s action to unilaterally cancel $500M in BARDA grants for mRNA vaccine R&D come after the U.S. government—through Secretary of Health and Human Services (HHS), Robert F. Kennedy Jr.—implemented several moves to weaken vaccine science and programming within the U.S. government:
- May 30: NIH notified the leaders of the Consortia for HIV/AIDS Vaccine Development (CHAVDs) that their funding would not be renewed, putting in doubt the realization of HIV vaccine portfolio and decades of historical investment.
- June 8: HHS Secretary, Robert F. Kennedy Jr removed COVID-19 vaccine recommendations for children and pregnant women and then dismissed 17 members of the CDC’s Advisory Committee for Immunization Practices (ACIP) and installed noted vaccine skeptics in their place.
- June 25: In a video statement, RFK Jr. stuns the Gavi replenishment conference by withdrawing U.S. support for the multilateral agency, critically undermining its ability to deliver vaccines to vulnerable populations and communities around the world.
- July 28: Following the decision made by the U.S. Supreme Court in Braidwood vs. Becerra, which affirmed the mandated insurance coverage of preventative services based on recommendations made by the U.S. Preventative Services Task Force (USPSTF) as well as the ability of the HHS Secretary to remove and shape HHS committees at will, RFK Jr. cancelled the next meeting of the Task Force and signaled his intention to disband its membership.
Cancellation of research grants at the CHAVD and BARDA continue a dangerous pattern of impoundment tactics by the administration to bypass Congressional authority to appropriate funding. Even with the potential restoration of funding, damage to the vaccine research infrastructure has already been done.
“We cannot just ‘turn the tap’ of funding back on,” warned Stacey Hannah, AVAC’s Director of Research Engagement. “When the administration stops research funding abruptly, it rewinds scientific progress. It will take time and even more resources to get these studies back online—squandering the potential of future breakthroughs that are based on established, gold-standard science.”
Additionally, by destabilizing evidence-based policymaking and scientifically rigorous recommendations made possible through ACIP and USPSTF, the administration intensifies public doubt and mistrust in vaccines as important public health tools. Vaccine misinformation undermines delivery of the annual flu shot, COVID-19 boosters, and vaccines against measles, hep B, HPV, and mpox, amongst other vaccine preventable diseases.
“These actions dangerously sow vaccine disinformation and mistrust, which has proliferated since the COVID-19 pandemic,” said Alison Footman, Senior Program Manager for STIs at AVAC. “Dangerous ideology results in dangerous policymaking, putting many lives at stake and complicating efforts to both discover and implement clinical and cost-effective interventions to make America and the world healthier, safer, and more prosperous.”
AVAC calls upon Congress to re-assert its power of the purse and its long-standing, bipartisan support for vaccine R&D and vaccination programs, to counteract efforts by the current administration, and to sustain the vaccine enterprise before it is too late.
###
About AVAC: Founded in 1995, AVAC is an international non-profit organization that provides an independent voice and leverages global partnerships to accelerate ethical development and equitable delivery of effective HIV prevention options, as part of a comprehensive and integrated pathway to global health equity. Follow AVAC on Bluesky and Instagram. Find more at www.avac.org and www.prepwatch.org.
Avac Event
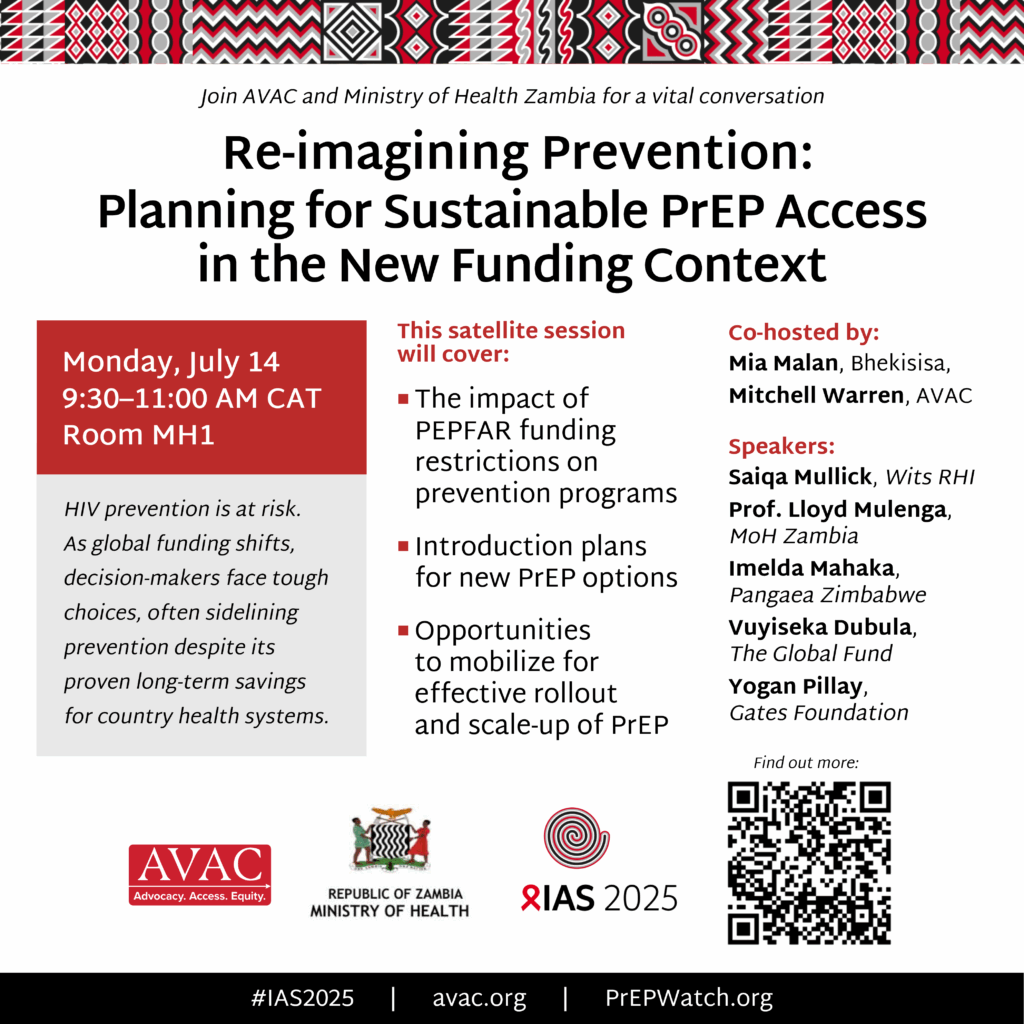
Re-Imagining Prevention: Ensuring sustainable PrEP access in an evolving funding context
If you are attending the International AIDS Society Conference (IAS), be sure to join AVAC and the Ministry of Health, Zambia for this vital conversation.
Avac Event
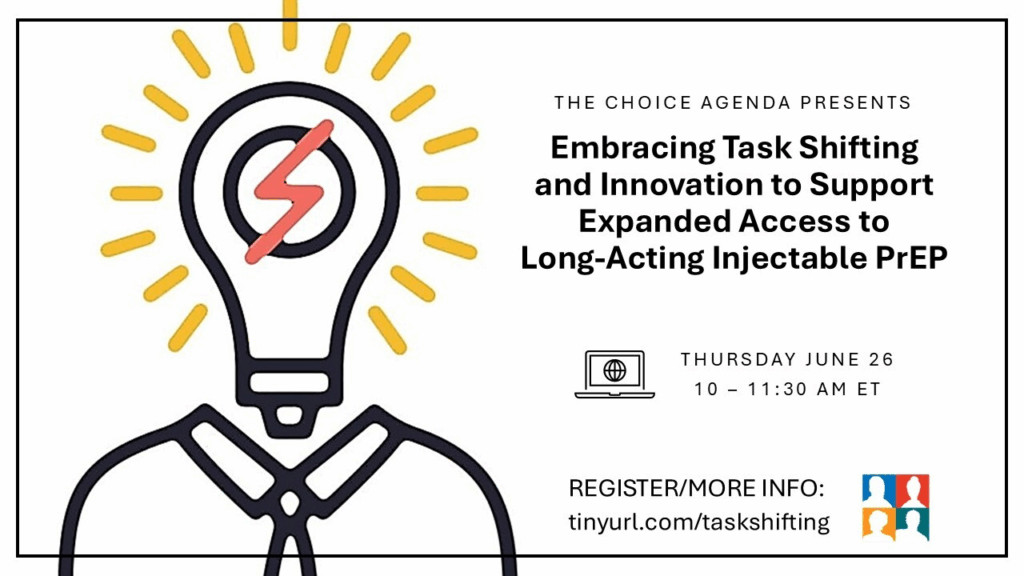
Embracing Task Shifting and Innovation to Support Expanded Access to Long-Acting Injectable PrEP
While the HIV prevention buffet will soon offer a second form of long acting injectable PrEP, ensuring access to all those who can benefit requires innovations in service delivery such as task shifting. In the United States, two Federally Qualified Health Centers (FQHCs) have implemented programming that has expanded clinic capacity, resulting in more individuals being able to choose long acting injectable PrEP. We also heard about innovative efforts to expand PrEP access in South Africa and learned what it takes to integrate task shifting for long-acting PrEP injection programs. We discussed other ways we can collectively innovate to support expanded, sustainable access to all forms of PrEP.
Speakers:
- Kevin Aloysius, Legacy Community Health, Houston
- Megan Dieterich, Whitman-Walker Health, Washington, DC
- Juan Carlos Loubriel, Whitman-Walker Health, Washington, DC
- Carey Pike, Desmond Tutu Health Foundation, Cape Town
Moderator:
- Rupa Patel, Washington University in St. Louis
Materials:
The Scientific Journey of Lenacapavir — and the Urgency to Defend HIV Prevention Science
On June 11, AVAC hosted a conversation, The Scientific Journey of Lenacapavir: From basic science to clinical development to impact, to explore how US support from NIH for basic science and South Africa’s clinical research infrastructure made possible the development of lenacapavir for PrEP (LEN), a discovery in HIV prevention that went on to be named Science magazine’s 2024 Breakthrough of the Year.
As the field anticipates initial regulatory approval from the US FDA by June 19 and a WHO recommendation in July, Linda-Gail Bekker of the Desmond Tutu Health Foundation, Wes Sundquist of the University of Utah and Mitchell Warren of AVAC underscored how this moment of promise is threatened by sweeping attacks on science, research and the very systems that made the development of LEN possible.
Press Release
AVAC Condemns Removal of the Advisory Committee on Immunization Practices
Contact: [email protected]
NEW YORK, NY, June 11, 2025—AVAC strongly condemns Secretary of Health and Human Services, Robert F. Kennedy, Jr., for removing all members of the Advisory Committee on Immunization Practices (ACIP). This committee of vaccine experts—with decades of experience in vaccine development, delivery and safety—is responsible for developing the country’s vaccine policies and recommendations for the Centers for Disease Control and Prevention (CDC). At a time when science is under political attack and vital programs are being defunded, AVAC stands with researchers, advocates, and communities calling for Congress to defend public health and unbiased science, which is essential to safeguarding the health of all Americans. The Secretary’s actions attack the integrity of ACIP membership and is a direct threat to public trust in our health systems and in the essential role of vaccines in disease prevention.
“Vaccines remain among the most powerful public health tools ever developed,” said Mitchell Warren, executive director of AVAC. “Vaccines have transformed the global response to infectious diseases, from smallpox to measles to COVID-19, and they are central to the vision of ending the HIV epidemic. At a moment when the US should be investing more in vaccine science, access, and public confidence, it is investing less and simultaneously undermining vaccines generally. Secretary Kennedy’s short-sighted and unceremonious removal of all ACIP members is an alarming escalation in this administration’s campaign to dismantle evidence-based health policy, science and research.”
ACIP’s long-standing commitment to base vaccine recommendations purely on the evidence represents the highest standards of ethical guidance to protect human health. The Secretary’s decision undermines not just US vaccine strategy, but global confidence in immunization programs and guidance that have long relied on US leadership.
The destruction of ACIP adds to the five-month litany of assaults on vaccines and the systems that support them. From proposed cuts to the Fiscal Year 2026 budget, to the defunding for global vaccine access programs like Gavi and domestic immunization initiatives at the US CDC, to the undermining of the essential role of measles vaccines, the closure of the leading NIH-funded HIV vaccine consortia (CHAVD), to the dismantling of USAID’s HIV vaccine R&D programs and the recommendation to remove COVID-19 vaccines from the US immunization schedule for children and pregnant women, this administration has worked to subvert the importance and impact of life-saving vaccines and erode public trust for vaccine science.
Vaccines work. Human papillomavirus (HPV) is responsible for 99% of cervical cancers. The HPV vaccine prevents over 90% of cancers caused by HPV, including anal, cervical, penile, throat, vaginal, and vulvar globally, and if more widely available, could prevent hundreds of thousands of annual deaths, according to the WHO. Similarly, hepatitis B (HBV) accounts for 1.1 million deaths globally, and yet the HBV vaccine prevents as many deaths every year, according to WHO. Furthermore, without a vaccine review panel, rollout of anticipated vaccines that protect against gonorrhea, could make access difficult for many Americans when drug resistant gonorrhea is on the rise globally.
“We are witnessing other countries eliminate cervical cancer through robust HPV vaccination and screening programs while the US risks reversing decades of progress,” says Alison Footman, AVAC’s senior program manager of STIs. “The ACIP plays a critical role in ensuring vaccines, including those that prevent STIs like HPV and hepatitis B, are accessible, and recommendations are guided by expert opinions. Now more than ever, we must protect the integrity of public health systems that save lives and prevent diseases.”
As public confidence in vaccines erodes, the value of vaccine science is paramount, representing one of the single greatest advances in the history of medical science, eradicating once life-threatening infections and mitigating the risk of illness from numerous diseases. In the field of HIV, the search for an effective vaccine is advancing thanks to decades of investment in basic science, clinical research, and global partnerships. This progress must be protected and accelerated. A vaccine would provide a durable, scalable form of HIV prevention that does not rely on frequent adherence or health system access, and it would be especially transformative for communities most marginalized by current systems.
AVAC calls on Congress, scientists, and civil society to speak out and stand up for science, for vaccines, and for the future of global and public health.
###
About AVAC
AVAC is an international non-profit organization that provides an independent voice and leverages global partnerships to accelerate ethical development and equitable delivery of effective HIV prevention options, as part of a comprehensive and integrated pathway to global health equity. Follow AVAC on Bluesky and Instagram. Find more at www.avac.org and www.prepwatch.org.