This market assessment supports countries, donors, implementing partners, and advocates in making informed decisions about the introduction, scale-up, and equitable delivery of long-acting PrEP among key populations and other priority groups.
Long-Acting PrEP Market Assessment for Key Populations
Press Release
FDA Approves Injectable Lenacapavir for PrEP
A Historic Milestone Must Now Be Matched by Urgent Action
Contact: [email protected]
New York, NY, June 18, 2025 — AVAC welcomes the U.S. Food and Drug Administration (FDA) approval of injectable lenacapavir (LEN) for the prevention of HIV as pre-exposure prophylaxis (PrEP). LEN, developed by Gilead Sciences, is a twice-yearly injectable PrEP option that showed nearly complete protection against HIV in the landmark PURPOSE 1 and 2 trials. Science Magazine named LEN the “Breakthrough of the Year” in 2024, a recognition that reflects its enormous potential. But that promise will only be realized if it is rolled out with speed, scale, and equity.
“The approval of LEN is a much-needed boost for HIV prevention, given the strength of the science and the simultaneous disruption in HIV programs globally,” said Mitchell Warren, executive director of AVAC. “But US FDA approval is just one in a series of steps needed to ensure that injectable LEN can help reduce the 1.3 million new HIV infections that occur each year. Scientific progress only matters if innovation actually reaches people. LEN for PrEP is poised to re-shape the HIV response, but only if today’s approval is accompanied by bold, strategic, effective and equitable rollout that reaches the populations that need access. Otherwise, the world risks squandering this PrEP opportunity, as it has with other PrEP options too often over the past 12 years.”
In December, PEPFAR and the Global Fund announced a coordinated ambition to reach two million people within three years of product launch. This commitment signals an unprecedented opportunity to make PrEP access a reality. But translating this ambition into impact, especially now amid the current political environment, is not without considerable challenges.
“Political will, programmatic implementation, and sustainable funding are needed to truly accelerate equitable and impactful introduction of LEN worldwide,” said Wawira Nyagah, AVAC’s director of product introduction & access. “We have over a decade of hard-won lessons on what it takes to rollout PrEP effectively, and the field cannot afford the delays we have seen with the past launches of daily oral PrEP, the monthly dapivirine vaginal ring (DVR), and every-two-month injectable cabotegravir (CAB). Lives depend on speed, scale and equity.”
The World Health Organization (WHO) is expected to release updated PrEP guidelines for LEN in July, and regulatory agencies in Brazil, Europe and South Africa are simultaneously reviewing the product. But the current political context, including a shuttered USAID and further disruptions across global health, demands an urgent and courageous response. In January, the US Administration issued a stop-work on all USAID-funded grants, nearly paralyzing HIV treatment and prevention by PEPFAR, the primary funder of programs in HIV-burdened countries (and administered by USAID). In February, PrEP was broadly excluded from a waiver that allowed HIV treatment to continue and allowed PrEP only for pregnant and breastfeeding women. These policies could not only undercut LEN’s promise but roll back years of progress in HIV prevention.
It will take new, re-vitalized and committed partnerships to work together to sustain past progress and advance HIV prevention to deliver on the UN targets for epidemic control. AVAC’s The Gears of Lenacapavir for PrEP Rollout outlines the steps needed from national governments, funders, researchers, drug-makers including generic manufacturers, and civil society to ensure LEN reaches those who need it most. In the near term, these stakeholders each have vital work to do to complement the initial announcement from the Global Fund and The Children’s Investment Fund Foundation (CIFF) in their pledged collaboration to significantly expand access to LEN for PrEP.
“No one donor, national government or manufacturer can realize this ambition alone,” said Warren. “All stakeholders—including Gilead, PEPFAR, and the Gates Foundation—must act decisively to seize this opportunity, ensuring that all populations—regardless of geography, income, or identity—benefit from this innovative prevention option.”
Meeting this moment requires funders, Ministries of Health, implementers and civil society partners to collaboratively design a comprehensive introduction strategy that breaks the sequential nature of traditional approaches to scaling up interventions. Instead, to speed up introduction, stakeholders must move toward a parallel approach where research, implementation science, and programs at scale are designed, funded and implemented simultaneously. This introduction strategy should entail:
- Other funders and national governments to join the Global Fund and CIFF and commit to procure at least enough LEN from Gilead for two million person years of protection beginning this year through 2027.
- Gilead to set a cost-effective price that compares to generic daily oral TDF/FTC. Achieving this will require a low launch price from Gilead, significant volume procurement from donors, and the entry of multiple generic manufacturers into a competitive, multi-million-user market. While this low price is not expected at launch, stakeholders must act now to reach this price point as quickly as possible by building volume with supplies from Gilead at no more than $100 per person per year and to support multiple generic manufacturers to enable production at larger scale and lower prices as quickly as possible.
- A mobilized civil society in high-burden countries pushing national governments to expedite regulatory approvals, integrate LEN into HIV and national health programs with domestic resources, and develop national guidelines without delay.
- Civil society also demanding transparent pricing and a clear, accelerated pathway to sustainable PrEP programs—so that by the time generic LEN becomes available around 2028, the market is primed for rapid scale-up, with multiple producers driving down prices through competition.
- Gilead working with their generic license holders to accelerate production and expand generic availability in middle-income countries.
- The US Administration, via the State Department, releasing all appropriated funds, negotiate best prices at scale and provide LEN to all who need it. These actions are essential to achieve a strategic transition and sustainability against the global HIV epidemic.
“This is the moment to build on the momentum of science, which has brought the field to this day, when LEN for PrEP is speeding through regulatory review faster than any prevention product to date,” said Nyagah. “Translating this success into real impact on the epidemic, led by communities around the world, must be a top priority among all stakeholders.”
###
About AVAC: Founded in 1995, AVAC is an international non-profit organization that provides an independent voice and leverages global partnerships to accelerate ethical development and equitable delivery of effective HIV prevention options, as part of a comprehensive and integrated pathway to global health equity. Follow AVAC on Bluesky and Instagram. Find more at www.avac.org and www.prepwatch.org.
Global Health Watch: WHA78, Misinformation at Congressional Hearings, Global Fund Cuts & More
The US Secretary of State and the Secretary of Health and Human Services appeared before Congress this week defending foreign aid cuts and the dismantling of USAID. Advocates are responding, including the Treatment Action Group (TAG) which issued a stark warning: US agencies are engaging in “unethical, dishonorable, and potentially law-breaking machinations” under new leadership, particularly at the NIH. Meanwhile, the US was absent from the World Health Assembly, where the WHO Pandemic Agreement was ratified and where a high-level dialogue on long-acting HIV prevention took place. All this plus looming Global Fund shortfalls, and new COVID-19 vaccine policy changes in this week’s issue.
Misinformation and Controversy at Congressional Hearings
The US Secretary of State Marco Rubio appeared in front of the US Congress this week defending radical cuts to foreign aid and a proposed State Department reorganization that deprioritizes global health programs. In front of the Senate Foreign Relations Committee (of which he was once a member), Rubio was corrected when he wrongly claimed that only 12% of US funding goes to direct services. In fact, 12% is the proportion of funds going directly to local NGOs, with 85% of total funding going towards direct services. He also claimed – without evidence – that 85% of PEPFAR beneficiaries were still receiving services and denied any deaths linked to the US cuts. The Secretary repeated these claims before the House Foreign Affairs Committee and Appropriations State and Related Programs subcommittee and ignorantly characterized voluntary male medical circumcision (VMMC) as wasteful spending. VMMC is proven to reduce transmission of HIV. Congressional members challenged Secretary Rubio on the legality of the foreign aid freeze and dismantling of USAID and highlighted reported deaths resulting from the cuts. Meanwhile, some Republican Senators, including Senate State, Foreign Operations, and Related Programs (SFOPs) Chair Lindsay Graham, expressed support for foreign assistance amidst calls for transparency and metrics to transition countries off US funding.
Department of Health and Human Services (HHS) Secretary, Robert F. Kennedy Jr. appeared before the Senate this week to defend cuts to public health programs and biomedical research. Similar to his appearance last week before the Senate Health, Education, Labor and Pensions (HELP) Committee, Kennedy’s remarks were controversial and contradicting.
IMPLICATIONS: These hearings underscore the challenges facing US global health policy and programming amid shifting political priorities and leadership. Advocacy to counter mis- and dis-information, and a vision for this new era of global health financing, are more important than ever.
READ:
- Senate Democrats Grill Defiant Rubio on Trump Policies—The New York Times
- Rubio: ‘No children are dying on my watch’—Devex
An Ethical and Legal Crisis
In a statement, TAG demands immediate action by the NIH to provide Congressionally appropriated and committed funding to the HIV clinical research networks (ACTG, HPTN, HVTN, IMPAACT).
World Health Assembly Updates
The United States was notably missing from the World Health Assembly (WHA) this week, with no official delegation attending. This is a major change from previous years where delegations held leadership and diplomacy roles. In contrast, China sent more than 180 delegates and pledged an additional $500 million over the next five years to help stabilize the agency following the withdrawal of the United States, reinforcing its growing influence in global health governance.
Meanwhile, the WHA formally voted to adopt the WHO Pandemic Agreement, a legally binding accord that lays the foundation for future pandemic prevention and response, including real-time sharing of vaccines, treatments, and diagnostics. For three years, member states negotiated critical issues, with pressure from civil society to embrace key provisions on equity and intellectual property. Some of those provisions have been addressed, but negotiations on an annex detailing the new Pathogen Access and Benefit Sharing (PABS) mechanism will continue with the aim of concluding at next year’s WHA. PABS refers to a proposed system where countries share genomic information about novel pathogens and share tools developed to combat those pathogens, regardless of which country discovered the pathogen or developed effective tools—it has been one of the most contested areas of negotiation. Though the treaty is less ambitious than earlier drafts, nations at the WHA have largely welcomed the agreement as a major achievement.
Many events and discussions were held on the sidelines of the WHA, including Wednesday’s high-level dialogue organized by the Global HIV Prevention Coalition (GPC). The event, A New Era of HIV Prevention: Accelerating Access to Long-Acting Prevention Options, was co-hosted by UNAIDS, in collaboration with the United Nations Population Fund (UNFPA), United Nations Development Fund, (UNDP), WHO, the Federal Republic of Brazil and Kingdom of the Netherlands. AVAC’s Mitchell Warren, who also co-chairs the GPC, opened the session with urgency and optimism, calling it “one of our greatest opportunities in 44 years of HIV prevention,” even as global solidarity is waning. Dr. Lilian Benjamin Mwakyosi, a past AVAC Fellow and director of DARE in Tanzania issued a powerful reminder that “choice for HIV prevention is not a luxury. It’s a right,” stressing that adolescent girls and young women need accessible, discreet prevention options like long-acting PrEP. The dialogue underscored the need for political will, financing, and community-centered action to turn scientific breakthroughs like lenacapavir for PrEP into sustained prevention at scale.
READ:
- United States Appears Set to Skip World Health Assembly while China Sends Over 180 Delegates to Geneva—Health Policy Watch
- For the first time, the U.S. is absent from WHO’s annual assembly. What’s the impact?—NPR
- A Pandemic Treaty Without Teeth Will Leave Africa and the World Exposed—Think Global Health
- Presidents and Prime Ministers Celebrate the Passing of the Pandemic Agreement—Health Policy Watch
Global Fund Financial Challenges
More than 260 civil society advocates joined a conversation organized by the Coalition to build Momentum, Power, Activism, Strategy & Solidarity (COMPASS), Eastern Africa National Networks of AIDS and Health Service Organizations (EANNASO), CHANGE, and others to explore the financial constraints facing the Global Fund following its Board meeting earlier this month. Advocates learned unmet pledges and declining global health aid will mean that the current Global Fund Grant Cycle 7 will reduce allocations to countries to align with available resources, moving from pledge-based to cash-based allocations. Countries will receive reduced funding envelopes in mid-June 2025, which will trigger a two-week reprioritization process to focus on life-saving services like treatment continuity and community-led monitoring while deferring lower-priority items.
IMPLICATIONS: These changes could jeopardize essential programs, especially those supporting key populations. And they also raise significant questions about the recently launched Global Fund replenishment for the next grant cycle. Civil society must prepare now to advocate for transparent processes, protect vital community interventions and support the Global Fund’s ambition to introduce injectable lenacapavir with speed, scale and equity.
New US COVID-19 Vaccine Policy
The US Food and Drug Administration (FDA) outlined in a new blueprint for COVID-19 vaccines, one that shifts from a recommended annual COVID vaccine for everyone 6 months and older, to adults over 65 and individuals with high-risk conditions, such as compromised immune systems. This shift requires vaccine manufacturers to conduct extensive clinical trials before approving vaccines for healthy individuals aged 6 months to 64 years, potentially delaying access for this group. Rather than proposing the new guidelines through the typical regulatory processes, including opportunities for public comment, this framework was devised and published by the head of the FDA along with the new head of FDA’s Center for Biologics Evaluation and Research (CBER).
IMPLICATIONS: This unorthodox process could complicate future vaccine approvals, as well as leave interpretation and coverage decisions up to insurers, which would create major access barriers for many.
READ:
- An Evidence-Based Approach to Covid-19 Vaccination—New England Journal of Medicine
- FDA will limit Covid vaccines to people over 65 or at high risk of serious illness, leaders say—STAT
New Resources: Tracking the Impact of US Cuts to Foreign Aid, USAID, and Research
As the US administration continues to dismantle foreign aid infrastructure and retreat from its commitments to science and global health, AVAC is tracking the impacts and consequences.
- Worldwide Prevention, Shared Protection outlines the global threat posed by defunding STI research and programming.
- What Happened to PEPFAR? provides an in-depth look at the stop work orders and contract terminations disrupting HIV prevention access.
- HIV Prevention R&D at Risk highlights how US policy shifts are endangering the future pipeline of HIV prevention tools.
What We’re Reading
- The case of the minister and the HIV activists: Are we entering denialism 2.0?—Bhekisisa
- China to donate $500 million to WHO, stepping into gap left by U.S.—Washington Post
- Trump Lectures South African President in Televised Oval Office Confrontation—The New York Times
- Disaster Awaits Us if the NIH Dies—The Nation
- Lambda Legal Sues National Institutes of Health over Terminating Critical Research Grants Relating to LGBTQI+ Health—Lambda (statement)
- Exclusive: NIH grant rejections have more than doubled amid Trump chaos—Nature
- PEPFAR’s Golden Era Is Over. It Urgently Needs a Five-Year Transition Plan—CSIS
Resources
- Politics and Global Health: The Need for a New, Resilient Architecture—Oxford University
- U.S. Funding Cuts Threaten 39 Research Sites in South Africa Putting Scientific Advancements and Hard Won Progress Against TB and HIV at Risk—TAG and MSF
- Advocates’ Guide: Understanding the President’s Proposed Fiscal Year 2026 (FY26) Budget and Its Implications for Science, Research and Global Health—AVAC
- Critical Advocacy: How Civil Society is defending the HIV Response and Global Health—AVAC
- Research Matters—AVAC, HIVMA, TAG
- Impact of NIH Grant Terminations—Association of American Medical Colleges
Long-Acting PrEP Real-Time Data Dashboard
The GBGMC dashboard provides updated insights on the effectiveness of Long Acting PrEP interventions, user demographics, and access rates across regions. Visit here.
Avac Event
100 Days In: How HIV Advocates are Meeting the Moment
In its first 100 days, the Trump administration proposed deep cuts to public health and HIV funding, attacked evidence-based healthcare, defunded scientific research, rolled back protections for LGBTQ+ people, and emphasized punitive criminal legal approaches. These moves pose serious threats to the future of HIV-related services, care, prevention, and the broader struggle for health equity and racial justice in our multiracial democracy.
Join CHLP for this moderated panel discussion focused on what the first 100 days of the Trump administration have meant for our communities, particularly people living with HIV, Black and brown people, LGBTQ+ people, and those impacted by criminalization, and how we are collectively shifting strategy to meet the current political moment.
Panelists
- Michael Elizabeth, Equality Federation
- Venita Ray, Black South Rising
- John Meade, PrEP in Black America, AVAC
- Chauncey McGlathery, American Academy of HIV Medicine
- Jada Hicks & Sean McCormick, CHLP
Why HIV Prevention Must Not Be Left Behind
In this presentation at the INTEREST 2025 conference, Rhoda Msiska of Copper Rose Zambia emphasizes the urgency of protecting the progress made in scaling up PrEP and the need to act now to expand access to new HIV prevention tools like injectable lenacapavir (LEN) and the Dual Prevention Pill (DPP).
Advocates’ Guide: Understanding the President’s Proposed Fiscal Year 2026 (FY26) Budget and Its Implications for Science, Research and Global Health
The US administration’s proposed Fiscal Year 2026 (FY26) budget marks a sweeping rollback of federal investment in health, research, and global development. For advocates, researchers, and implementers, this proposal demands urgent attention and action.
This initial “skinny budget” is a proposal and not yet law. A more detailed proposal will be released by mid-to-late May and the US Congress will ultimately decide actual funding levels for FY26, which begins October 1. So, advocates must speak up now to protect funding for research and programming that saves lives and livelihoods.
Here’s what advocates need to know and do:
Big Picture: A Dramatic Retrenchment
The budget proposes $163 billion in cuts to non-defense discretionary spending, including a 26% reduction to the Department of Health and Human Services (HHS)— the department that oversees the US National Institutes of Health (NIH), the Centers for Disease Control and Prevention (CDC), and the Food and Drug Administration (FDA). These cuts are completely offset by an increase to defense spending and reflect a shift toward the elimination of science and programming tied to diversity, equity, inclusion (DEI), gender, and climate, and a redirection of funding toward defense and “America First” priorities—priorities that put the perceived interests of the US and its citizens over other national and global issues.
Detailed Analysis and Implications
Health and Biomedical Research
The proposed cuts to HHS would gut federal support for health and biomedical research, dismantling key programs at NIH and CDC. They threaten progress on infectious diseases, health equity, and pandemic preparedness—undermining decades of scientific gains and leaving communities vulnerable.
NIH is Cut by $17.9 billion losing HIV and global health research
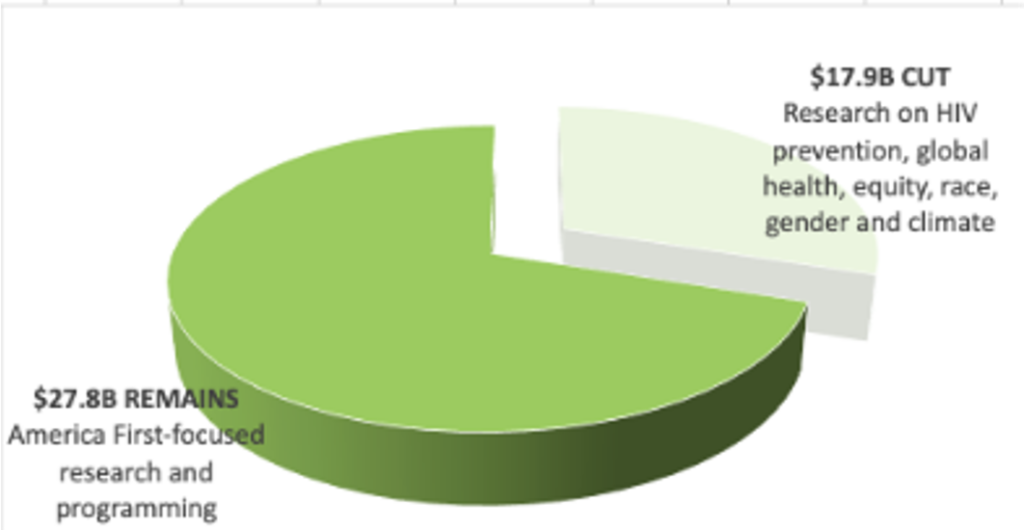
- Preserves $28 billion of the $46 billion for NIH overall, but excludes HIV prevention, global health, and health equity research.
- Reorganizes NIH into 5 “realigned” institutes, removing focus on climate, gender, racial equity.
- Eliminates the Fogarty International Center and the National Institute on Minority Health and Health Disparities.
Centers for Disease Control and Prevention (CDC): Cut by $3.59 billion
- Eliminates Global Health Center and National Centers on environmental health, injury prevention and chronic disease prevention.
- Eliminates DEI programs and shifts the burden for pandemic prevention and response.
Agency for Healthcare Research and Quality: Effectively eliminated
- Cited as redundant; targeted for work on climate and gender.
National Science Foundation (NSF): Cut by $4.9 billion (56%)
- Eliminates funding for work seen as ideologically objectionable (e.g., broadening participation and racial equity in STEM).
Global Health and Development
At a time when the USG should be expanding access to new technologies, the proposed FY26 budget guts foreign assistance funding, threatening pillars of the global HIV response: the President’s Emergency Plan for AIDS Relief (PEPFAR) and US contributions to multilateral initiatives, such as Global Fund and GAVI. The ideological targeting of family planning and gender-related programs will further weaken interventions to address HIV, which have been shown to work best within a comprehensive package of health and social services.
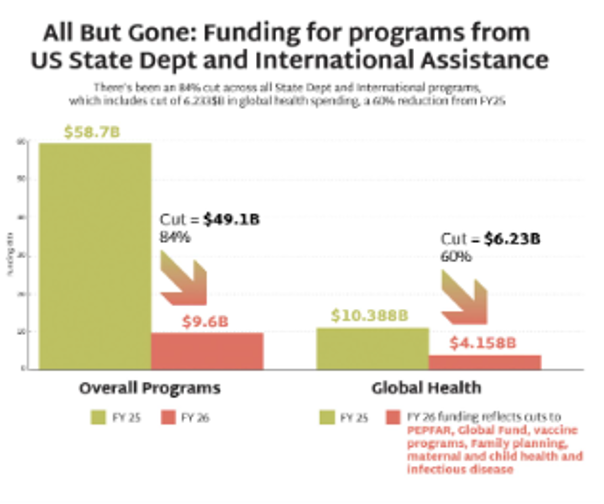
Global Health Programs: Cut by $6.23 billion
- Defunds NGOs providing family planning, impacting maternal and child health providers.
- PEPFAR preserved only for existing treatment programs and programs for the prevention of mother-to-child transmission (PMCT) , and specifically excludes primary prevention and PrEP, except for pregnant and lactating populations.
USAID Development Aid: Cut by $8.33 billion
- USAID is eliminated with the limited number of existing programs moved into the State Department.
- Eliminates DEI, climate, and gender-related programming.
- Creates new “America First Opportunity Fund” to replace foreign assistance grants with loans that prioritize US interests over humanitarian needs.
Centers for Disease Control and Prevention (CDC)
- STI, TB, hepatitis programs folded into a reduced $300 million block grant.
- For more on the specific impacts on STI programs, please read AVAC’s new brief on Worldwide Prevention, Shared Protection: Why STI Funding Matters.
Health Resources and Service Administrations (HRSA): Cut by $1.73 billion
- Ryan White HIV/AIDS Program activities not deemed core are eliminated.
Substance Abuse and Mental Health Services Administration (SAMSHA): Cut by $1.065 billion
- Eliminates harm reduction and regional substance use program grants.
Offices of Minority & Women’s Health
- Moved under a new, less visible structure.
New Initiative: “Make America Healthy Again”
- $500 million focused on lifestyle over treatment.
What This Means
- HIV Prevention R&D and global implementation is at risk. Cuts to NIH and USAID directly threaten support for clinical trials, community engagement, and biomedical innovation.
- Equity-centered research threatened. Eliminating institutes focused on minority and global health severely undermines inclusive science and jeopardizes future impact. Inclusion is not just a nice to have, it’s integral to achieving impact
- PEPFAR protections are narrow. Only existing beneficiaries are covered; scale up and innovation are excluded, compromising the imminent introduction and potential impact of injectable lenacapavir for PrEP. Funding for HIV prevention is also eliminated, except for pregnant and lactating populations.
Advocacy Priorities
- Monitor the full FY26 budget release for agency-level detail and justification.
- Engage Appropriations and other relevant Committees via coalition efforts (e.g., FAPP, GAPP, GHTC, SHF).
- Mobilize your community to contact your Senators and Representatives to let them know you oppose these cuts.
- Share your stories from researchers affected by cuts—particularly those whose work is globally focused or funded by NIH/USAID.
- Stay up to date with budget briefings and mobilization opportunities. See AVAC’s ‘Research Matters’ resource, which shares guidance and a toolkit for researchers to advocate for continued funding.
This budget is a threat to decades of progress in science, equity, and health—but it is also an opportunity to speak with clarity and urgency about what is at stake. Advocates must ensure that the future of HIV prevention, global health innovation, and equitable science is not written by politics, but by people, evidence, and impact.
PrEP Delivery Imperiled
Programs for delivering PrEP have been shuttered all over the world by the withdrawal of the US government from global health. This graphic illustrates some of the severe measurable impacts of these cuts. Excerpted from PxWire.
PxWire Volume 15, Issue 2
The field of HIV prevention is confronted with two opposing forces; programs for delivering PrEP have been shuttered all over the world by the withdrawal of the US government from global health. At this same moment in history, next-generation long-acting products hold great promise to accelerate HIV prevention and help the world achieve epidemic control. Navigating these seismic developments requires unprecedented coordination, solidarity, and courage.
Global health champions can defy the hatred, fear, and greed that are dominating politics in so many places around the world. Together we can innovate, create, and protect the advance of HIV prevention and global health. This issue provides a snapshot on threats to delivering PrEP, the potential of injectable lenacapavir (LEN) for PrEP, and on the implications of upstream research and development of other long-acting PrEP.
Read below or download the PDF version.
Progress in PrEP Uptake: Threatened
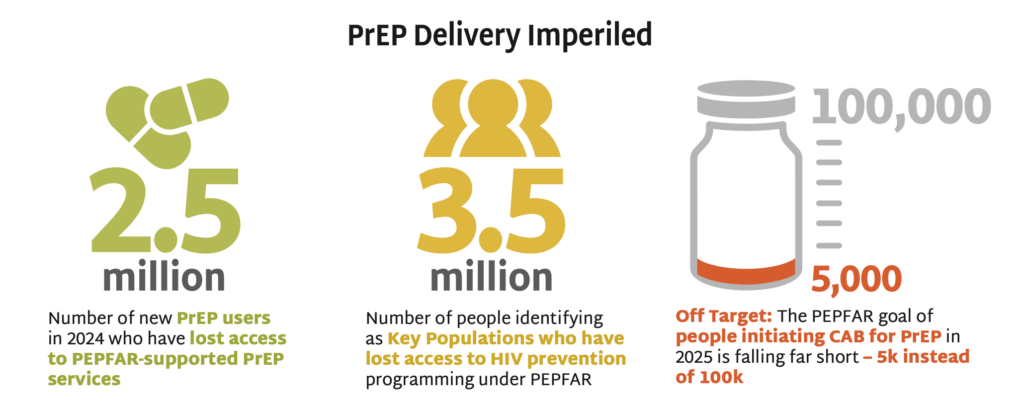
- PEPFAR documented 2.5 million new PrEP users in 2024, who could now lose access to PEPFAR- supported PrEP services. US Department of State issued a limited and inconsistently implemented waiver in February, allowing for continued provision of HIV treatment but restricting PrEP access to pregnant and lactating people only.
- These actions will result in 3.5 million who identify as key populations (KPs) losing access to all HIV prevention programming under PEPFAR, according to 2024 PEPFAR data tracking KP use of PrEP. These groups have higher rates of HIV incidence and face additional barriers to accessing services now that targeted programs are suspended.
- PEPFAR had a goal of 100,000 people initiating cabotegravir (CAB) for PrEP in 2025. But only 5,000 individuals had initiated by October 2024. The suspension of PEPFAR funding imperils scale-up of this long-acting product.
- These figures represent just some of the disruptions that are decimating PrEP delivery. Learn more here: Impact of PEPFAR Stop Work Orders on PrEP
- Overcoming this challenge, restoring, sustaining, and accelerating PrEP access is imperative and possible if the field works together.

For the last eight years, AVAC has proudly worked with PEPFAR to document PrEP uptake and its impact around the world. That stopped in January with a stop work order from the US government. But protecting access to PrEP is vital. Are you leading a PrEP program? Whether supported by PEPFAR or not, we invite you to work with us to ensure global data on PrEP is not lost. Find us at [email protected].
PrEParing for New Products
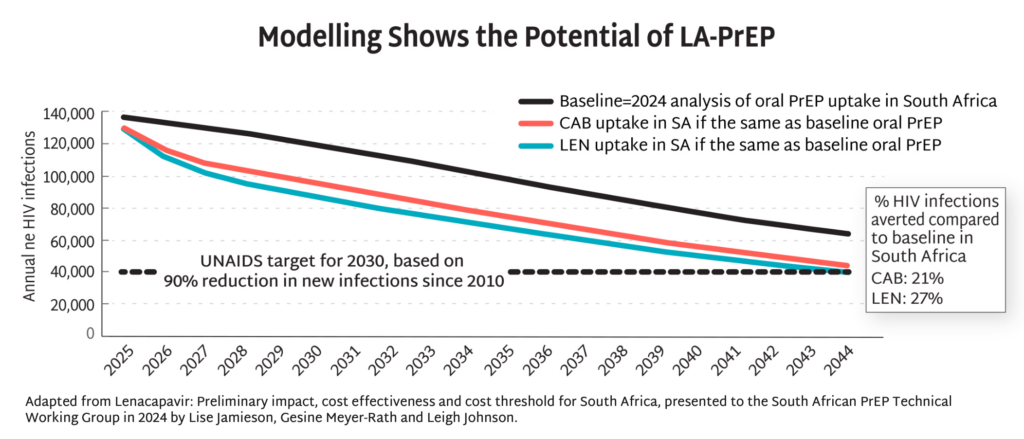
- Approval by the US Food and Drug Administration (FDA) for injectable 6-month LEN for PrEP is expected in June, with WHO guidelines expected in July. See the full timeline.
- Modelling data from South Africa demonstrate the potential of injectable PrEP to dramatically reduce HIV incidence by up to 90% by 2044, and potentially even sooner with more aggressive uptake. This potential goes beyond South Africa, lighting the way toward epidemic control the world over.
- The field must be prepared for swift action once LEN is approved and recommended, to ensure this opportunity is not squandered. As AVAC’s interactive timeline, Tracking LEN Rollout, outlines, donors, ministries of health, manufacturers, regulators, and civil society all have a role to play to pave the way for swift, equitable and effective introduction of LEN for PrEP.
The Latest R&D in the Prevention Pipeline
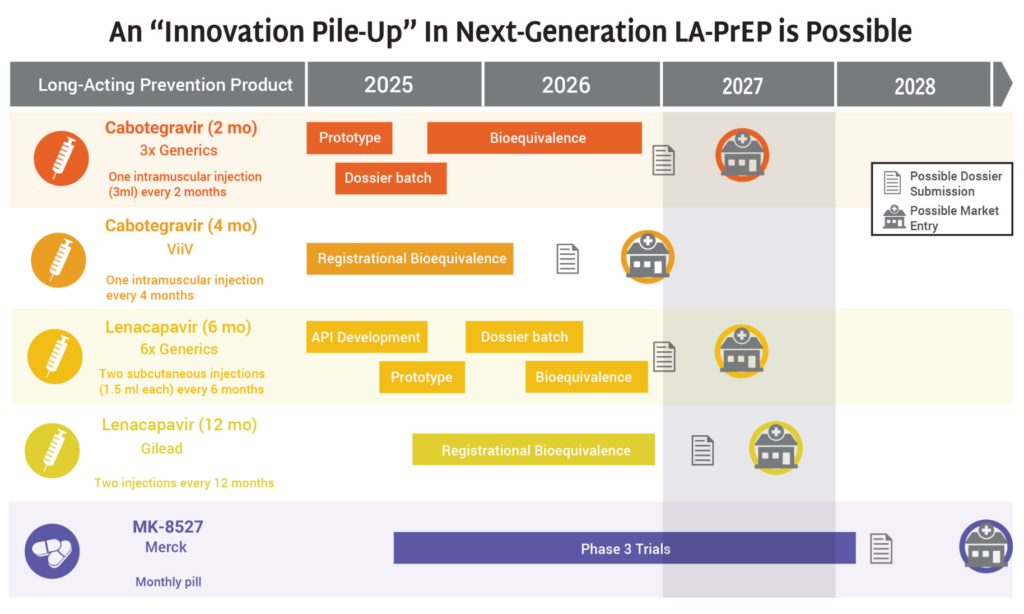
- The HIV prevention market is headed toward a period of significant opportunity—and possible congestion—as a slate of new products are on track for continued development and potential introduction to the market in 2027 and 2028.
- Generics for 2-month CAB and 6-month LEN, along with ViiV’s 4-month CAB, Gilead’s 12-month LEN, and Merck’s monthly oral MK-8527 PrEP pill (if further development and approvals are successful) could all enter the market by 2028.
- The possibility of so many products on the market, including four different formulations of injectable PrEP, means that it is imperative the field prepares for this future now.
- Markets and policies must be built to support the products in the market already, so that new options can be rapidly deployed and deliver impact. Otherwise, the field will squander time and money, with epidemic control slipping further out of reach.
- With US funding cuts to both HIV prevention R&D and delivery, communities must be engaged, supported, and informed about all prevention options, and the choices that all stakeholders will need to make. This means gathering and sharing data and information about cost-effectiveness, user acceptability, program feasibility, and impact. Communities empowered with the facts can advocate for the choices they need, and push ministries of health to make strategic investments and procure the prevention method mix that fits their context and delivers impact.
Prevention Playlist
AVAC develops a wide range of resources to inform decision making and action. Check out the latest:
Join
- Subscribe to Global Health Watch: AVAC’s weekly newsletter dedicated to breaking down critical developments in US policies and their impact on global health, at avac.org/global-health-watch
- CHANGE: In response to the unfolding crisis, more than 1,500 people from civil society organizations around the world have launched CHANGE—Community Health & HIV Advocate Navigating Global Emergencies—a coalition formed to support urgent action: [email protected]
Use
- Research Matters Advocacy Toolkit, AVAC
- Tracking the Freeze: Real-Time Impact on Key Populations, GBGMC
- Impact of the Stop-Work Order on PrEP, AVAC
- Tracking Lenacapavir Rollout, AVAC
- PEPFAR Program Impact Tracker, Impact Counter
- Weekly Situation Report, UNAIDS
Watch/Listen
- Politics and Global Health: The Need for a New, Resilient Architecture, Webinar
- Lawsuit Wins and What’s at Stake: AVAC v US Department of State, PxPulse episode
- Global Health in the Lurch: What’s happening now and who is pushing back, PxPulse episode
- Advancing Sexual and Reproductive Health and Rights: Moving forward post-the 2024 US Election, Webinar
Read
- Despite USG Global Health Collapse, Here Are Several Data Trackers To Support Your Advocacy, AVAC
- On Going Backwards, Lancet
- The Trump Administration’s Foreign Aid Review: Status of PEPFAR, KFF
- The Best Investment You Didn’t Know You Made: How NIH Funding Fuels Innovation and Economic Growth, amfAR
- HIV Market Impact Memo, CHAI
- The USAID List of Terminated Global Health Awards – What Does it Tell Us?, KFF
- AVAC Condemns HHS Mass Layoffs
- PrEP in the Balance: Hopes and fears in 2025
- Better Engagement, Better Evidence: Working in partnership with patients, the public, and communities in clinical trials with involvement in good participatory practice, Lancet
- PEPFAR: A Strategic Necessity for US Leadership and Global Health, AVAC
- Rallying for HIV Prevention Amid Policy Attacks, AVAC
- CROI 2025 Shows the Promise of Research at its Best, AVAC
Avac Event
Science in the Crosshairs: Research Advocacy in a Time of Crisis
AVAC and partners had a critical conversation on the escalating threats to health research and equity-centered science. This webinar unpacked the implications of the proposed FY2026 US federal budget—which includes sweeping cuts to NIH, CDC, USAID, and the elimination of vital global and minority health research programs. Together, we explored what these attacks mean for communities, researchers, and implementers and identified actionable advocacy strategies to fight back.
Recording / Slides / Resources
