PxWire Volume 13, Issue No. 4
Takeaways
- This year, four countries started providing PrEP for the first time, and seven countries
exceeded 70,000 new PrEP initiations, most of which are attributable to PEPFAR. - As of Q3 2023, the top five West and Central African countries for PrEP initiation have surpassed 10,000 initiations.
- For the first time since oral PrEP was introduced in 2012, PrEP users will have a range of methods to choose from—but only if they have access to them.
- ViiV, the sole manufacturer of CAB for PrEP, announced a 40 percent increase in doses that could be available for non-commercial use through 2025, an increase to 1.2 million doses.
- HIV R&D is focused on ARV and non-ARV based prevention products and HIV vaccines that build on new knowledge about the virus.

PxWire is AVAC’s quarterly update covering the latest in the field of biomedical HIV prevention research and development, implementation and advocacy. Each issue includes updates, emerging issues and upcoming events. Also available as a PDF.
From Research to Rollout: A look at where we are in HIV prevention
As we look back on 2023, powerful crosscurrents confront HIV prevention and global health equity. More options than ever before could be available, but many forces threaten to undermine access to proven prevention options that exist today and the development of additional options that are still needed. Threats to PEPFAR and hate laws targeting LBGTQI+ people are just a sample.
Upstream research and development is dynamic, but robust stakeholder engagement and sustainable funding must still be secured and integrated into a people-centered research agenda. These commitments are the compass that will ultimately lead to impact in the real world. The highlights below provide a snapshot of key updates in Q3 of 2023 and resources to inform advocacy on these critical questions.
Progress in PrEP Uptake
These updates from AVAC’s Global PrEP Tracker explore trends in 2023 and highlight new data as of September 2023.
PEPFAR and PrEP
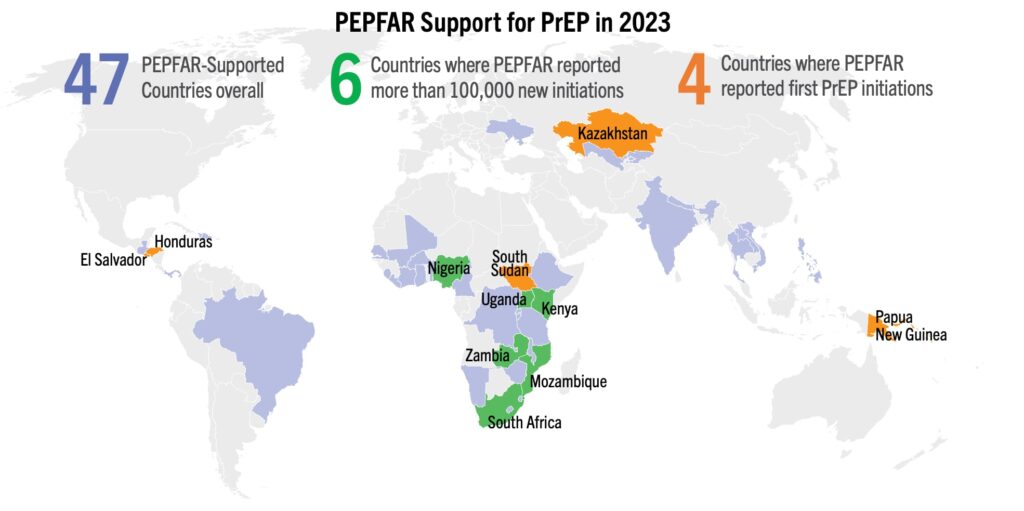
PEPFAR’s role has been instrumental to accelerating global uptake of PrEP to date. This year, four countries started providing PrEP for the first time, and seven countries exceeded 70,000 new PrEP initiations, most of which are attributable to PEPFAR. This lifesaving, uniquely effective, program must see continued full funding and a 5-year reauthorization to carry on this work and help to put the world on track to control the epidemic.
Cumulative PrEP Initiation Milestones
Global Milestones
- The world surpassed 5.6 million cumulative PrEP initiations as of September 2023. Initiations increased by 700,000+ since Q2 2023. Though comparable to previous quarterly increases, it is the highest ever recorded quarterly increase by the Global PrEP Tracker.
- At the same time last year the world had reached 3.3 million cumulative PrEP initiations, representing a doubling of initiations since then.
Country Level Milestones
- Early adopting African countries continue to show the most rapid increases in PrEP uptake. South Africa has shattered 1 million cumulative initiations. Nigeria, Uganda, and Zambia have all surpassed 500,000 initiations, Kenya has surpassed 400,000, and Zimbabwe has surpassed 200,000.
- Malawi and Brazil saw jumps of approximately 20,000 initiations over this quarter, while Lesotho had more modest increases of 10,000 initiations.
- Dozens of countries continue to grow their programs. For example, El Salvador, Honduras, and South Sudan all surpassed 1,000 cumulative initiations.
- Cyprus and Malta recorded their first oral PrEP initiations, at 29 and 579 respectively.
Spotlight on West Africa
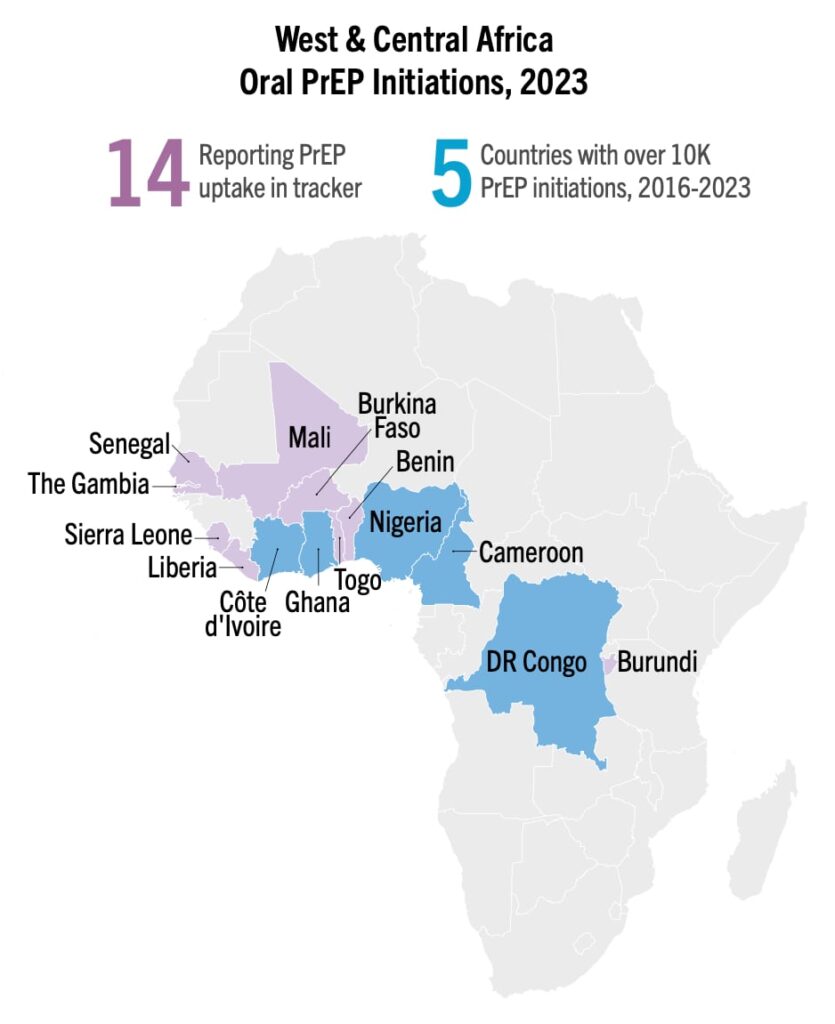
As of Q3 2023, the top five West and Central African countries for PrEP initiation have surpassed 10,000 initiations; Cameroon, Cote d’Ivoire, Democratic Republic of the Congo (DRC), Ghana and Nigeria. Nigeria has marked almost 550,000 cumulative initiations as of this quarter, ranking it second, behind South Africa, worldwide. These milestones can be credited to targeted PEPFAR investments. But despite these successes, West and Central Africa make up only 14 percent of PrEP initiations recorded in Africa, compared to 85 percent reported by East and Southern Africa. These same countries are home to two thirds of all people living with HIV in West Africa. There is vital work to be done to close the gap.
West and Central Africa can and must continue to leverage this recent growth in oral PrEP by making additional HIV prevention options available. In September, Nigeria’s regulators approved injectable cabotegravir (CAB for PrEP)—approving the first new biomedical HIV prevention option in a West and Central African country since oral PrEP. But at this time, only one other CAB for PrEP application has been submitted in the region, in Côte d’Ivoire, and none for the dapivirine vaginal ring (DVR). Only one CAB for PrEP implementation study is planned for the region.
PrEParing for New Products
Current HIV prevention options aren’t reaching all who need them. Access to a range of options that meet the needs of diverse people, especially those most at risk, will be essential to meeting global targets for ending the HIV epidemic.
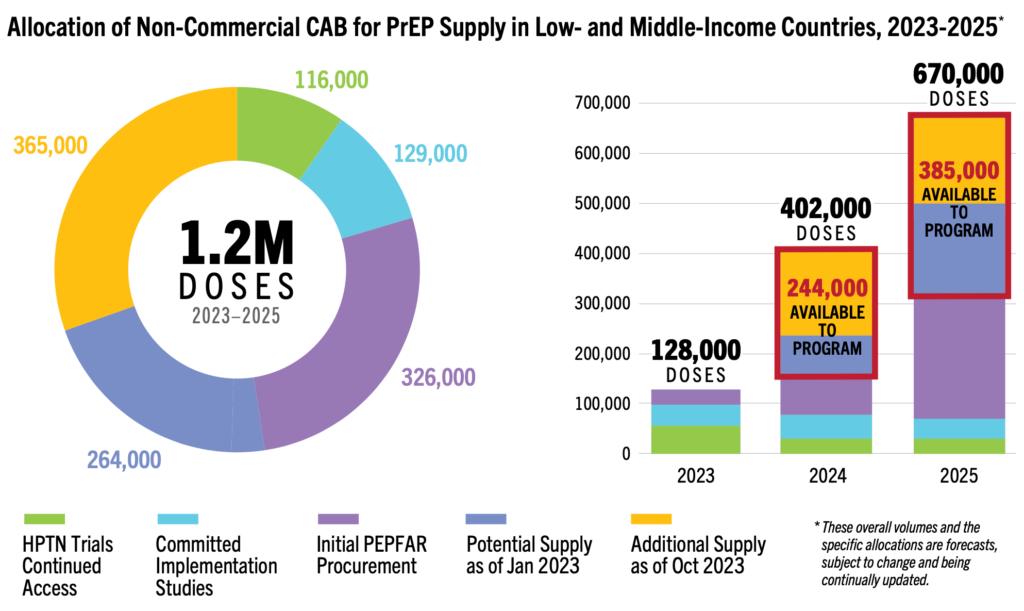
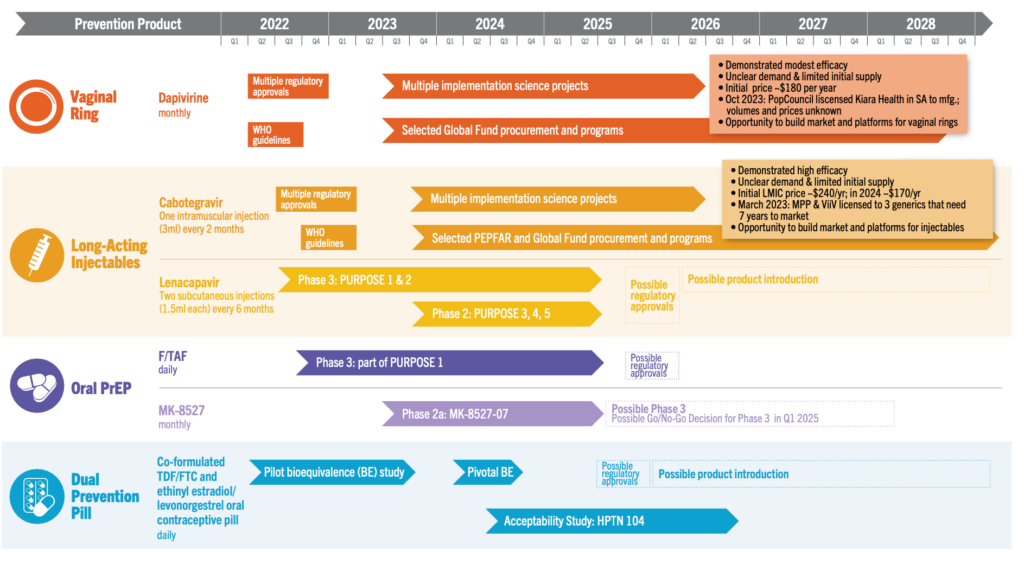
- CAB for PrEP Supply: In October 2023, ViiV, the developer and sole manufacturer of CAB for PrEP for now, announced a 40 percent increase in forecasted doses that could be available for non-commercial use in low- and middle-income countries through 2025, an increase to 1.2 million potential doses. Of these, 116,000 have been allocated to post-trial access for HPTN 083 and 084 participants; 129,000 for an initial set of eight implementation studies; 326,000 to PEPFAR programs in Malawi, Ukraine, Vietnam, Zambia and Zimbabwe; leaving at least 629,000 doses available for procurement by PEPFAR, Global Fund and national governments. See more details in our Country Planning Matrix. Three generic manufacturers could begin to deliver doses in 2026 or 2027.
- Implementation Science (IS): As DVR and CAB for PrEP supplies arrive in countries, dozens of implementation science studies are underway in 22 different countries. Check out AVAC’s Integrated Study Dashboard for more details and stay tuned for updates.
- PrEParing for Choice: For the first time since oral PrEP was introduced in 2012, PrEP users will have a range of methods to choose from—but only if they have access to them. Policy makers, donors, governments and implementers must commit to making these methods available, accessible and affordable. The HIV Prevention Choice Manifesto, launched in September by the African Women’s HIV Prevention Community Accountability Board, outlines what needs to happen to make choice a reality.
Approval Information
- Malaysia, Nigeria, Peru, Zambia, and the European Medicines Agency have approved CAB for PrEP; ViiV also made submissions in Canada, Colombia, and the United Kingdom. There are now 13 regulatory approvals and 15 additional submissions that are pending.
The HIV Prevention Pipeline: The latest in research, development and more
The HIV prevention pipeline has evolved: Few products are in late stage or efficacy trials, newly proven products are rolling out, and early phase clinical trials are exploring innovative strategies. R&D is focused on ARV and non-ARV based prevention products and HIV vaccines that build on new knowledge about the virus. The late-stage trials of recent years and basic science have brought deepening insights that are being applied today to a diversity of ‘upstream’ interventions.
- MK-8527 Announced: Merck announced a new Phase 2a trial to evaluate the safety, tolerability, and pharmacokinetics of a monthly oral pill, MK-8527. The trial began in November 2023 in participants at low-risk for HIV acquisition. If successful, Phase 3 trials could start in 2025.
- PURPOSE Program: A new Phase 2 study, PURPOSE 5, will evaluate lenacapavir as a twice-yearly prevention option in France and the United Kingdom, as part of the larger PURPOSE program. Purpose 5 is recruiting participants who are disproportionally affected and often underrepresented in HIV clinical trials. PURPOSE 1 and 2 are Phase 3 efficacy studies in Argentina, Brazil, Mexico South Africa, Thailand, Uganda and the United States. PURPOSE 3 & 4 are smaller studies focused on populations facing disproportionate risk.
- The Dual Prevention Pill (DPP): In September, the DPP, which would protect against pregnancy and HIV, moved to the next stage in R&D. A pilot bioequivalence study has shown the pill’s combined drugs—an antiretroviral and a contraceptive—are absorbed at an equivalent rate as taking them separately. The DPP now moves to a larger bioequivalence study needed for a regulatory submission. Check out the updated DPP Market Preparation and Introduction Strategy for highlights. The Population Council and Medicines360 are also developing a second-generation DPP with F/TAF.
Prevention Playlist
The most effective advocacy is based on smart analysis and accurate information. AVAC develops a wide range of materials and resources to inform decision making and action. Check out these essential resources to the global conversation on HIV prevention and global health equity.
JOIN
- ICASA: The biennial International Conference on AIDS and Sexually Transmitted Infections in Africa. Dec 4-9. Register
- How Our Environment Impacts Cure, webinar, Dec 13. Register
- Sex, Gender & HIV Cure Research, Dec 6. Register
READ
- Progress Against HIV and AIDS is Fragile, article
- STI Watch Newsletter, a curated resource on the latest STI vaccines, diagnostics and other prevention strategies.
- The STI Clinical Trials Dashboard, tracking trials on vaccines, doxycycline as post-exposure prophylaxis (DoxyPEP) and diagnostics against chlamydia, gonorrhea, hepatitis B, herpes simplex virus(HSV), human papillomavirus, syphilis and trichomoniasis infections.
- Paving the Road for STI Prevention Advocacy, blog
- Three HLMs, A Host of Challenges and One Major Victory, blog
- A Legacy of Impact: The power and reach of AVAC’s Fellows, report
- Advancing Choice in HIV Prevention Research, blog
- Adherence, Safety and Choice, Lancet article on MTN 034 offering DVR and oral PrEP to African women
- How ‘unauthorized status’ is threatening US global HIV initiative, article
- The GPP Body of Evidence, online clearinghouse
- From The Lab to The Jab, issue briefs
- Bending the Curve: What a decade-long rollout-out of the anti-HIV pill can teach the world, article
WATCH AND LISTEN
- The anti-HIV Jab is Coming to SA. Find Out When and How, podcast
- Spotlight on new PrEP tools & data, from R&D to access, webinar
- Africa Health R&D Week, webinar series
- Pandemic Accord Briefing for Civil Society, webinar
- PrEP Resources Showcase, workshop
- Let’s Talk HIV Research: An introduction to the science under investigation, webinar
- HPTN 096: Building Equity Through Advocacy—An integrated status-neutral approach for ending the epidemic among Black gay men in the South, webinar
- Inclusion of Pregnant and Lactating People in HIV Research: What you need to know, podcast
- Results from STI Landscaping Analyses in East and Southern Africa—Part 1 & Part 2, webinar
- Pioneering Self-care Solutions to Drive Access to HIV Prevention and Family Planning, webinar
- Boo, Syphilis is Really Back!, webinar
- Practicality over Panic: What happens if PEPFAR isn’t reauthorized?, webinar
- Foundations of HIV Cure Research, webinar
- PEPFAR at 20: Keeping the promise, podcast
Was this content helpful?
Tell us how we can improve the content.
Was this content helpful?
Thank you for your feedback!