Resources
AVAC’s Resource Database contains educational and advocacy materials covering a wide range of issues on biomedical prevention of HIV, STIs, COVID-19 and emerging health threats—from research to rollout.
To search for clinical trials and detailed information on products in development, visit our Prevention Research & Development Database

Results
showing 1-10 of 279
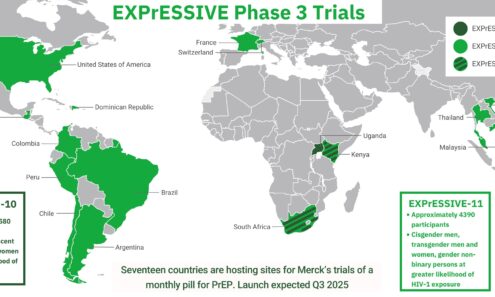
EXPrESSIVE Phase 3 Trials
Seventeen countries are hosting sites for Merck’s trials of a monthly pill for PrEP. Launch is expected in Q3 2025. This graphic shows where these trials are taking place.
Prevention Option:
Now What with Injectable LEN for PrEP?
The announcements on 9 July 2025 from Global Fund and Gilead about their next steps for injectable lenacapavir (LEN) for PrEP are welcome, as one more part of the process. But they raise as many questions as they answer. This brief summary is intended to help outline what is actually known–and not—and what needs to happen next.
Prevention Option:
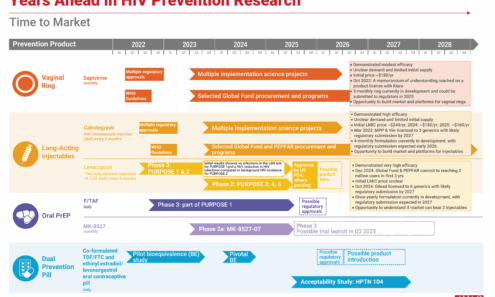
Years Ahead in HIV Prevention Research: Time to Market
This timeline shows the potential time points when the next-generation of HIV prevention options might find their way into new programs.
Prevention Option:
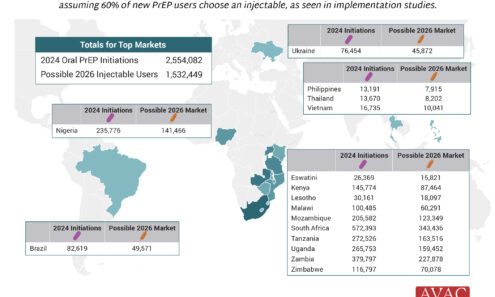
Potential Demand for LEN for PrEP
The top 16 PrEP markets, based on 2024 oral PrEP initiations and the possible 2026 injectable market, assuming 60% of new PrEP users choose an injectable, as seen in implementation studies.
Prevention Option:
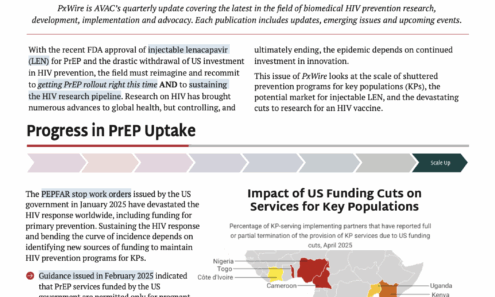
PxWire Volume 15, Issue 3
This issue of PxWire looks at the scale of shuttered prevention programs for key populations, the potential market for injectable LEN, and the devastating cuts to research for an HIV vaccine.
Prevention Option:
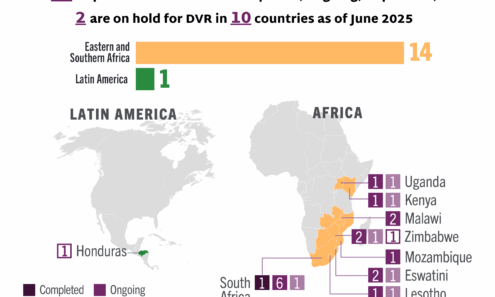
Dapivirine Vaginal Ring Implementation
Ongoing and planned implementation studies for the dapivirine vaginal ring as of June 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Prevention Option:
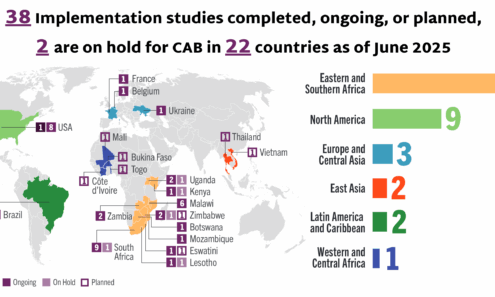
Cabotegravir Implementation
Implementation studies completed, ongoing, or planned for cabotegravir as of June 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Prevention Option:
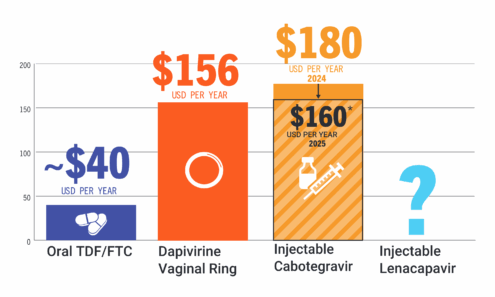
PrEP Price Comparison
Comparing the annual price of oral TDF/FTC vs. the dapivirine vaginal ring and injectable cabotegravir. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Prevention Option:
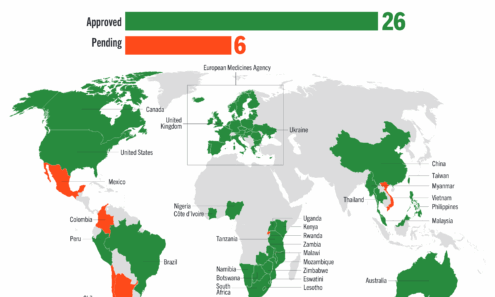
Dapivirine Vaginal Ring Regulatory Approval
Regulatory approvals, pending decisions, and appeals as of June 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Prevention Option:
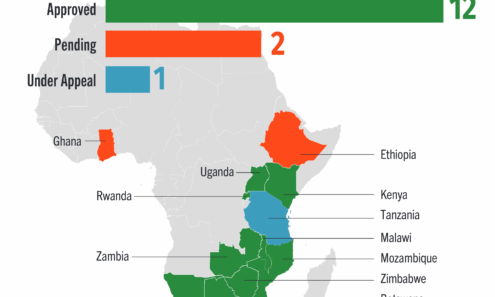
Cabotegravir Regulatory Approval
Regulatory approvals and those pending for cabotegravir as of June 2025. For product approvals, volumes, implementation, and price comparisons of long-acting PrEP, visit our dashboard on PrEPWatch.org.
Prevention Option:
showing 1-10 of 279