This week four Latin American countries have signed bilateral health Memoranda of Understanding (MOUs) with the US under its America First Global Health Strategy. This new Think Global Health analysis examines gaps between country needs and new co-financing commitments with the US.
In this issue, we also look at delays in the release of US science funding, despite congressional approval, and new developments from South Africa and AVAC shaping the global rollout of HIV prevention options.
US Appropriated Science Funds Remain Frozen Despite Feb. 3 Law
One month after the US Congress passed a $50 billion foreign affairs spending bill, which the President signed into law, funding has still not begun flowing to several agencies that support scientific research. According to Nature, the Office of Management and Budget (OMB) has not yet authorized the release of all appropriated funds, leaving agencies unable to issue new grants. As a result, the National Institutes of Health (NIH) has not yet received approval to spend the research funding allocated by Congress, prolonging uncertainty for scientists and institutions awaiting support for ongoing and new research projects.
IMPLICATIONS: While Congress ultimately rejected the deep budget cuts proposed by the President, the delays underscore the broad expansion of the power of the Executive Branch and the fragility of the current political environment in the US. As we wrote in our February 6 issue, “uncertainty of whether global health and humanitarian programs will receive the funding Congress appropriated leave many reluctant to celebrate. Coordinated advocacy and sustained Congressional oversight will be needed to ensure all appropriated funds are obligated and spent.”
READ:
- Trump Administration Funding Delays Worry NIH Grant Recipients—Bloomberg
- Foreign aid is back from the dead — but it’s in the hands of the people who tried to kill it—Vox
- White House stalls release of approved US science budgets—Nature
- Delays in awards and funding calls worry NIH-funded researchers—Science
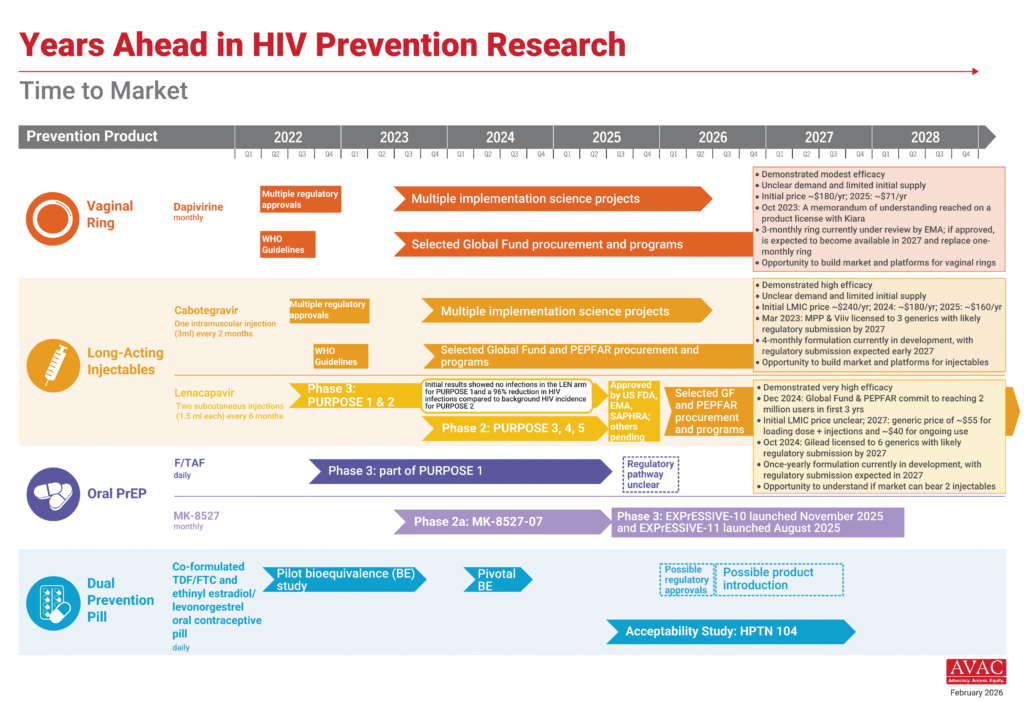
Lenacapavir Rollout Advances as South Africa Pursues Local Production
Momentum continues to build around the rollout of injectable lenacapavir for PrEP (LEN) across multiple countries. Four countries in East and Southern Africa have now launched LEN, with national launch events last week in Kenya and Zimbabwe. Eswatini and Zambia have already begun delivering LEN, and among the nine Global Fund to Fight AIDS, Tuberculosis and Malaria–supported early adopter countries, Lesotho, Mozambique and Uganda have also received initial supply. Nigeria is expected to receive shipments beginning next week. AVAC and the Coalition to Accelerate Access to Long-Acting PrEP continue to track the progress; see updated maps of regulatory approvals, initial programmatic access and implementation science.
In addition, this week, South Africa announced a new effort to enable local production of LEN, signaling growing interest in regional manufacturing. In October 2024, Gilead Sciences granted six voluntary licenses to generic manufacturers in Egypt (1), India (4) and Pakistan (1) to produce and supply generic LEN to 120 low- and middle-income countries. South Africa’s would mark a seventh voluntary license. As the majority of generic LEN is likely to come from Indian generic manufacturers (as four of six with licenses are from India), South Africa is looking to set up independent capacity and capability to be self-sufficient in the future.
IMPLICATIONS: The next phase of LEN rollout will depend on how quickly early launches translate into scalable, sustained access. A seventh license for a South Africa–based manufacturer could help accelerate the pathway from licensing to large-scale supply for regions most affected by HIV. Yet, it is not clear how long it might take the South African manufacturing to reach the market and at what price. In addition, the long-term sustainability of access remains uncertain amid broader disruptions to global health funding.
READ:
- Gilead and South Africa are negotiating a license for local production of new HIV drug—STAT
- Kenya rolls out breakthrough HIV drug. Now the real test begins—Defrontera
- South Africa launches bid to enable local production of long-acting HIV prevention drug, lenacapavir—Unitaid
AVAC Announces New Partner in Product Introduction
Beginning a decade ago, AVAC expanded its advocacy work to include a focus on accelerating product introduction and access. This week, AVAC announced the establishment of Access Bridge, an independent Kenya-based organization advancing timely, sustainable and equitable access to HIV prevention, sexual and reproductive health and related health products.
What We’re Reading
- Tracking the “America First” Bilateral Health Agreements—Think Global Health
- US Speeds up Signing of Bilateral Health Agreements, DRC Lawyers Challenge Minerals Deal—Health Policy Watch
- A titan of vaccine development sees his field’s achievements slip away—STAT
- Are faith-based organizations the future of the AIDS response?—Devex
- Africa CDC Flags Concerns Over U.S. Health Funding Deals—Birr Metrics
- Global health in a new era of US extraction—BMJ
- State Department eyes tuberculosis breakthroughs—Devex
- After US aid cuts, South Africa’s HIV response strains to hold the line—Devex
- Ghana and Senegal Consider Harsher Measures Against LGBTQ People—Health Policy Watch
- Community health project in Kenya and Uganda dramatically cuts new HIV infections—Science
- Inside the battle to end the Aids pandemic in the face of Trump’s cuts—The Independent
- The CDC is in chaos. But here’s where it’s devastating—Washington Post
- Turmoil Takes Hold at CDC as Top Officials Keep Leaving—Wall Street Journal
- NIH Says It Will No Longer Recognize the Research Fellows’ Union—Notus
- U.S. may be slowing rollback of vaccine policy ahead of midterms—HealthBeat
- The global fallout of RFK Jr.’s vaccine policies—NPR
- A new one-a-day-pill holds promise for HIV’s ‘forgotten population’—NPR
- States Move to Limit Access to H.I.V. Treatment—New York Times
- Impact of the US Global Gag Rules on LGBTQI+ People Abroad—Williams Institute
- Major US cohort reveals sharp racial disparities in HIV acquisition among trans women—Aidsmap