AVAC’s ‘One Year Later’ series reflects on the tumultuous events of the past 365 days across five global health issues. Our third piece is below. View the full series here.
The United States has witnessed a sustained assault on the research enterprise that underpins global health progress. Documented weekly in AVAC’s Global Health Watch, what has unfolded is a 365-day cascade of policy, programmatic and financial actions that threaten US leadership and the scientific ecosystem responsible for lifesaving breakthroughs from HIV prevention to pandemic preparedness.
In the first month of this US administration, approximately 1,800 National Institutes of Health (NIH) research grants were abruptly canceled, sending shockwaves through universities, research institutes, and global collaborations. In March, Health and Human Services (HHS) began to shrink its workforce by tens of thousands, with approximately 3,500 positions cut at the Food and Drug Administration (FDA), 2,400 at the Centers for Disease Control and Prevention (CDC), and 1,200 at the NIH as part of broader reductions and consolidations. Many scientists, recently hired staff, and early-career researchers lost their jobs, while senior leaders like Jeanne Marrazzo, then-director of the NIH’s National Institute of Allergy and Infectious Diseases (NIAID), and Kathy Neuzil, then-director of the NIH’s Fogarty International Center, were unceremoniously placed on leave or offered reassignment to distant posts as part of the reorganization. As Nature reports, there was an exodus of more than 25,000 people, many of whom were at early career stages across the science agencies. These disruptions hit core functions in disease surveillance, vaccine and drug development, and infectious disease research, and removed institutional capacity and decades of expertise needed to drive science and public health forward.
The administration then proposed restructuring of the NIH itself, alongside the President’s FY2026 budget request that proposed to cut $18 billion from its funding—a nearly 40% reduction. The administration’s proposal signaled a fundamental shift away from sustained investments in basic science and clinical research, the very foundation of discovery that enables future innovation across all health areas and diseases categories.
As AVAC has repeatedly warned, science does not operate with an on/off switch, it depends on sustained investment, stable institutions, and trust. “When the Administration stops research funding abruptly, it rewinds scientific progress. It will take time and even more resources to get these studies back online—squandering the potential of future breakthroughs that are based on established, gold-standard science.”
In September, the HIV research community responded to the assault on research: AVAC and partners convened 24 Hours to Save AIDS Research, a global, day-long virtual marathon featuring more than 70 scientists, clinicians, advocates, and community leaders to document what decades of federally supported HIV research have delivered—and what stands to be lost if investments continue to erode. Speakers shared firsthand accounts of how sustained NIH funding enabled breakthroughs in HIV treatment, prevention, and vaccine science, and warned that abrupt cuts threaten to dismantle research networks that cannot be easily rebuilt. The effort underscored that defending the research enterprise is essential to protecting lives, progress, and future innovation. Watch the videos here.
In November, HHS ordered the CDC to phase out so-called “non-essential” nonhuman primate research, jeopardizing preclinical studies that were central to the development of HIV PrEP and PEP, vaccines, and other critical interventions. Additional actions, including pauses on some international research collaborations, proposed caps on indirect cost rates that support university infrastructure, and changes to peer review processes, have further destabilized the system that ensures scientific rigor and independence.
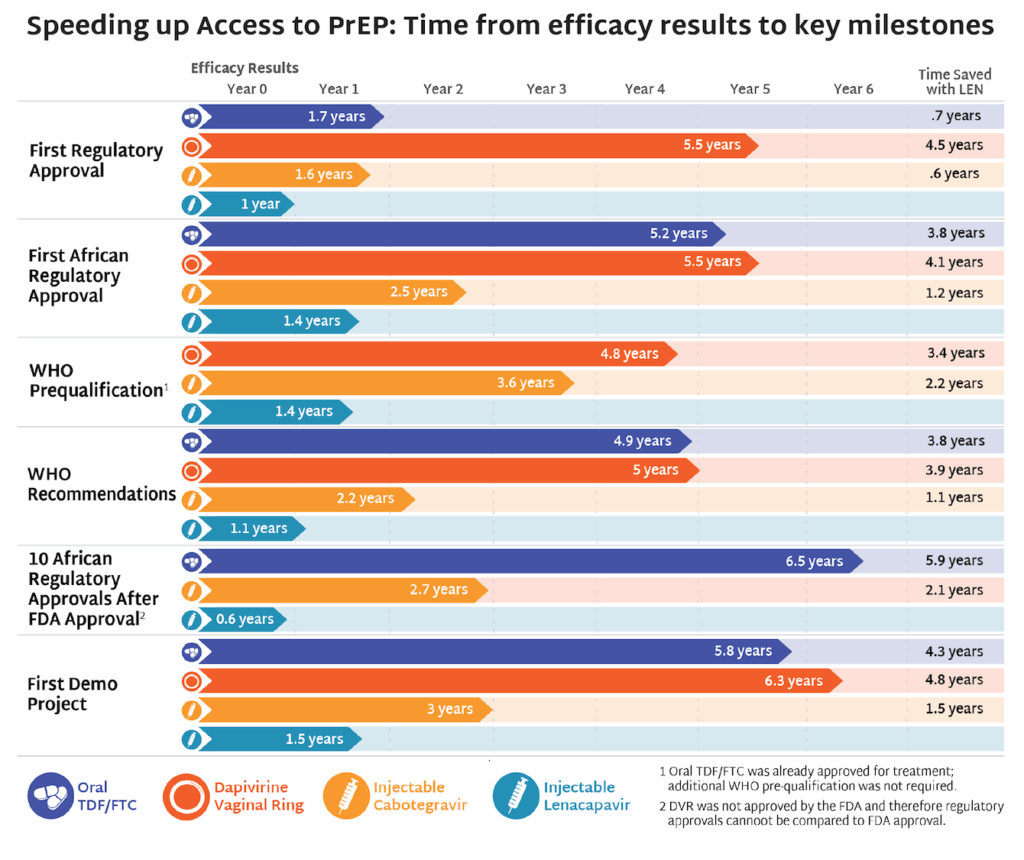
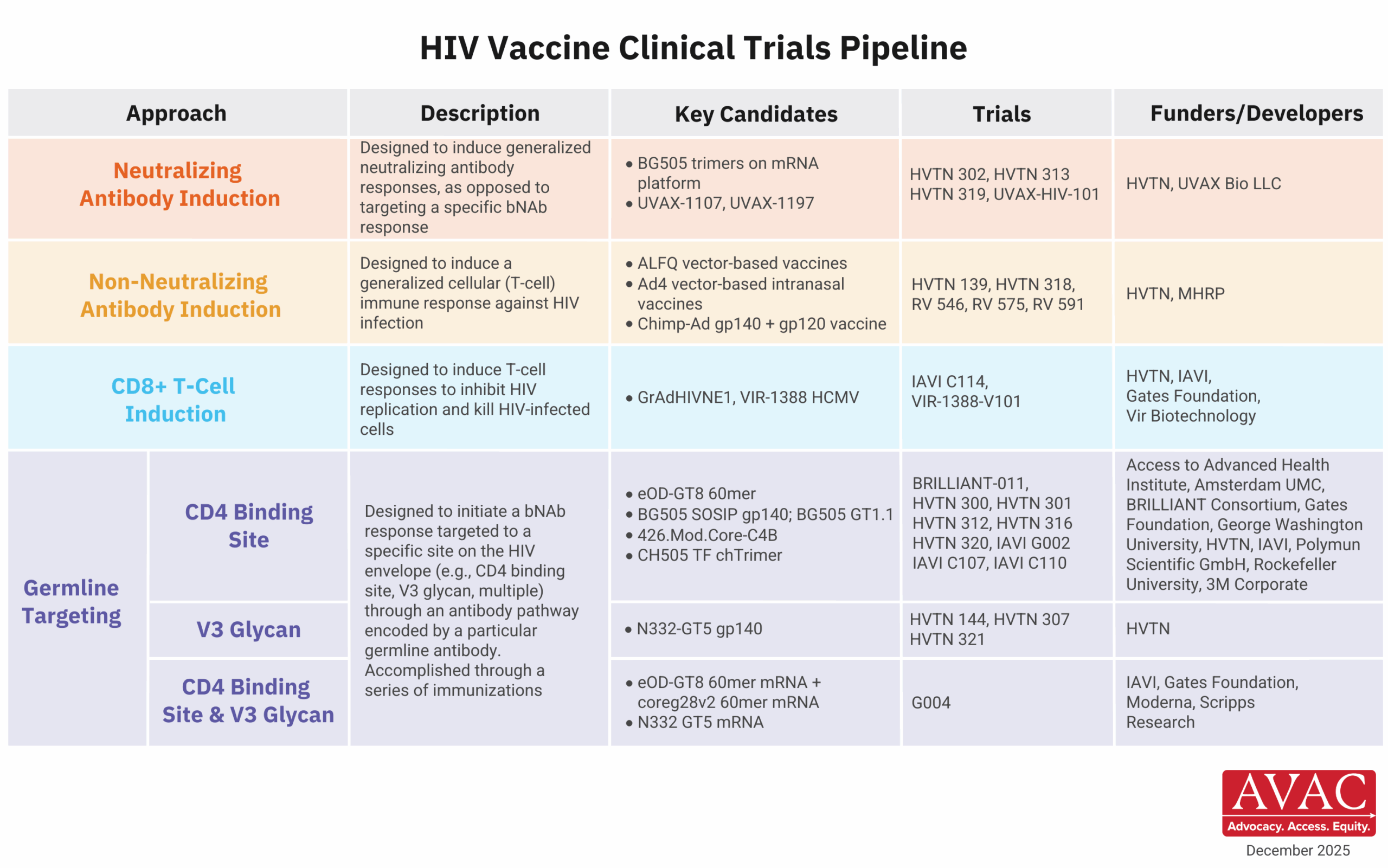
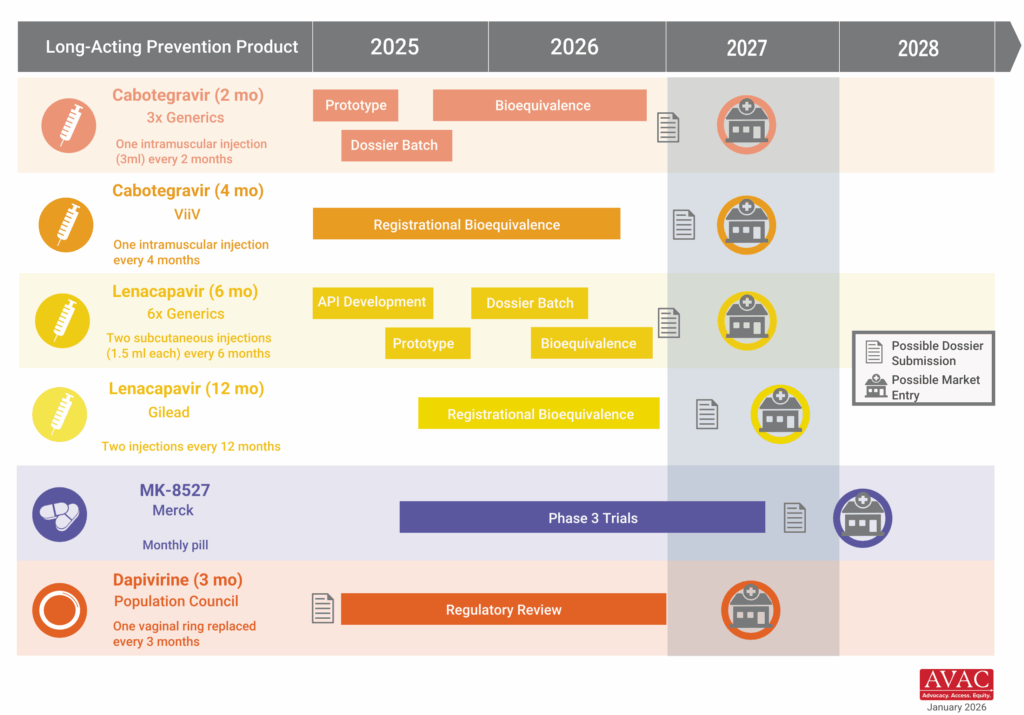
These developments come at a pivotal moment of extraordinary scientific promise. Long-acting HIV prevention options like injectable lenacapavir demonstrate what sustained investment can deliver when science is allowed to progress. As AVAC’s Mitchell Warren recently noted, “We are in a golden moment where innovation, evidence, and opportunity align—but breakthroughs only matter if the systems to deliver them remain intact.” Undercutting research infrastructure now risks slowing or derailing the next generation of prevention tools before they ever reach communities and deliver impact.
Meanwhile, Congress is in the midst of negotiations over the FY2026 appropriations process. While lawmakers on both sides have pushed back against the most severe cuts proposed in the Administration’s budget, the outcome remains uncertain. The decisions made in the coming months will determine whether the US can regain its leadership position or retreat at a time when global health challenges demand more, not less, scientific leadership.
The implications extend far beyond US borders. American investments have long anchored global research networks, supported scientists around the world, and accelerated progress against HIV, TB, malaria, and emerging threats. Dismantling that infrastructure weakens global preparedness, fuels mistrust in science, and leaves communities everywhere more vulnerable.
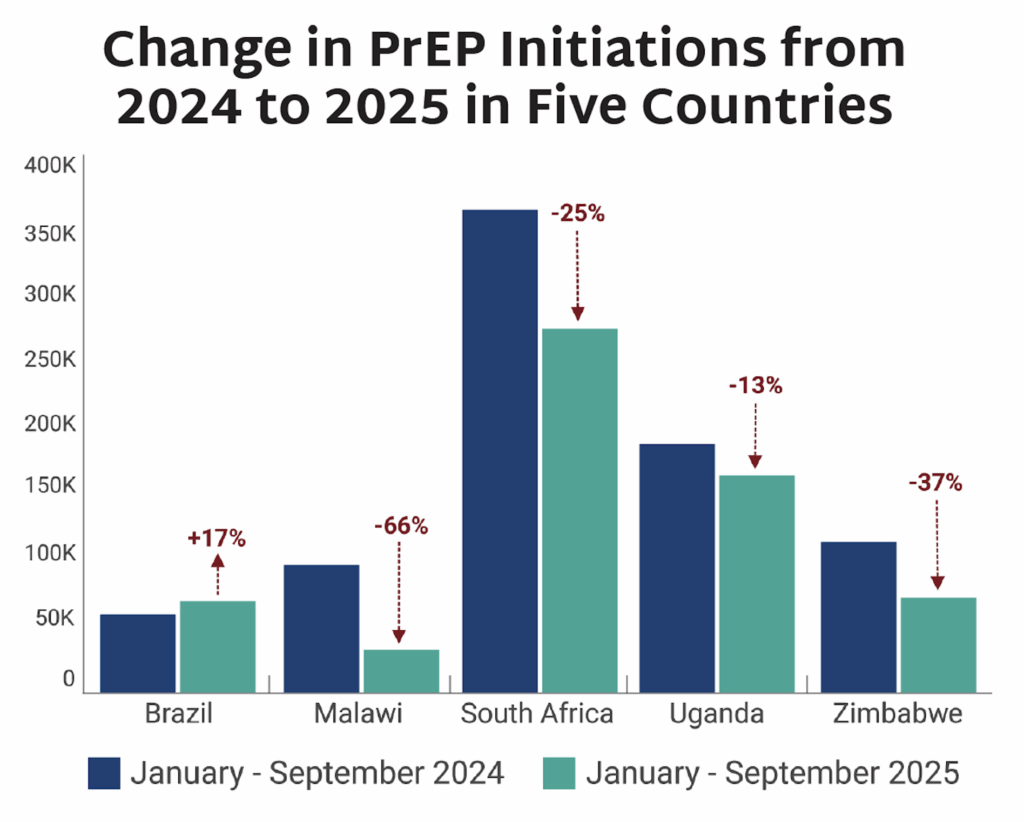
NIH funding, which supports an estimated 60-70% of South Africa’s medical research, significantly disrupted South Africa’s biomedical research enterprise. In March, NIH grant managers were told to “hold all research awards” for the country, labeling South Africa as a “country of concern” and halting sub-awards and freezing or blocking new and existing grants. A briefing in May shared that 27 HIV trials and 20 TB trials would likely be affected. “We will see lives lost. In excess of half a million unnecessary deaths will occur because of the loss of the funding, and up to a half a million new infections,” Linda-Gail Bekker told Al Jazeera. “Though the issues related to research cuts are a global challenge, South Africa does bear the brunt of so much of this new US presidential administration’s ire,” Mitchell Warren told the Financial Mail. “It is very clear that the President is using budgets and terminations of previously agreed to programmes to redefine agendas, and in this case, ones that defy science and are clearly political and ideological.”
Evidence, equity, and public health must remain guiding principles. AVAC will continue to track, analyze, and elevate what’s at stake, because defending the research enterprise is essential to saving lives. The future of HIV prevention, vaccine development, and global health depends on it.