By Jeanne Baron
Enormous changes are underway in HIV prevention and across global health. Global advocates know that we play an indispensable role as these changes take shape. It’s our work to vigilantly track new agreements, new policies and new investments in research, development and delivery. It is our voices that call out inequity, greed, misinformation, and misguided assumptions. Here are three questions we advocates are asking along with resources to support our collective work.
Q1: How do we speed access to LEN for PrEP and why is building a sustainable market a critical next step?
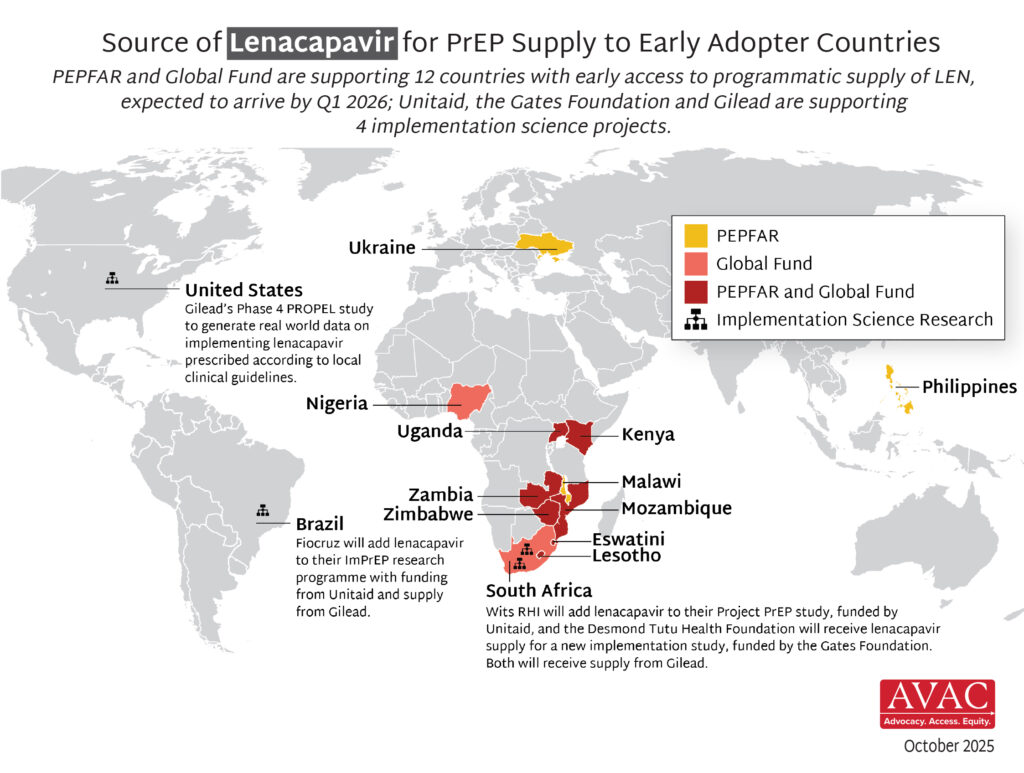
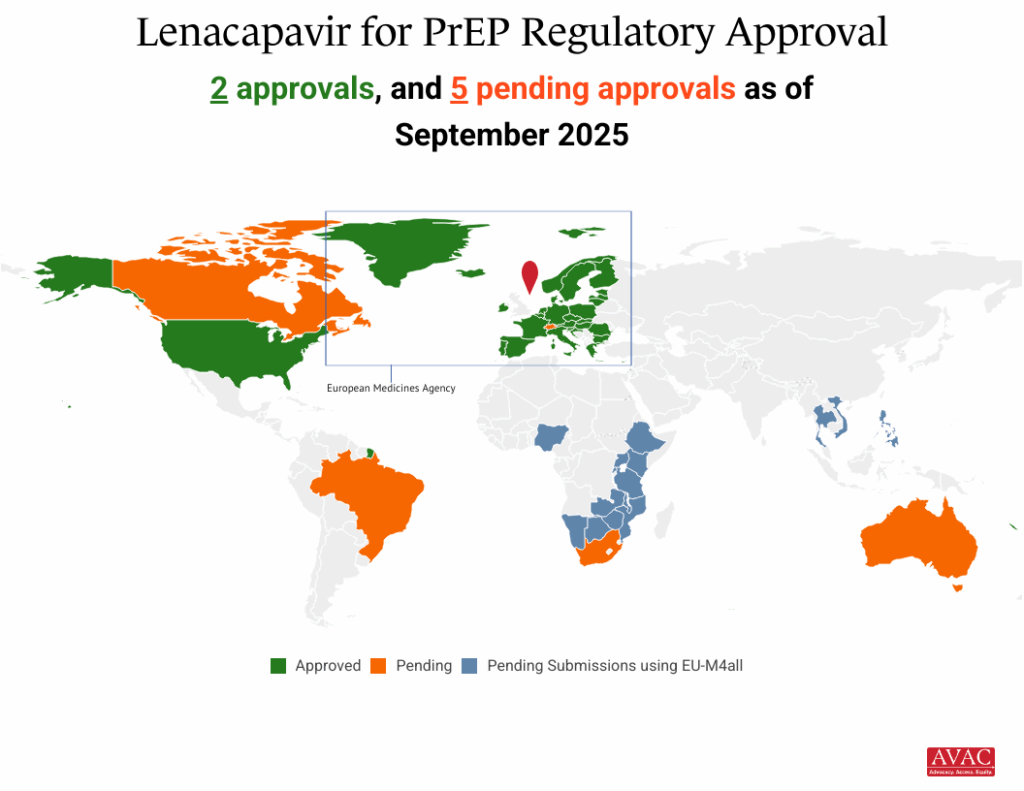
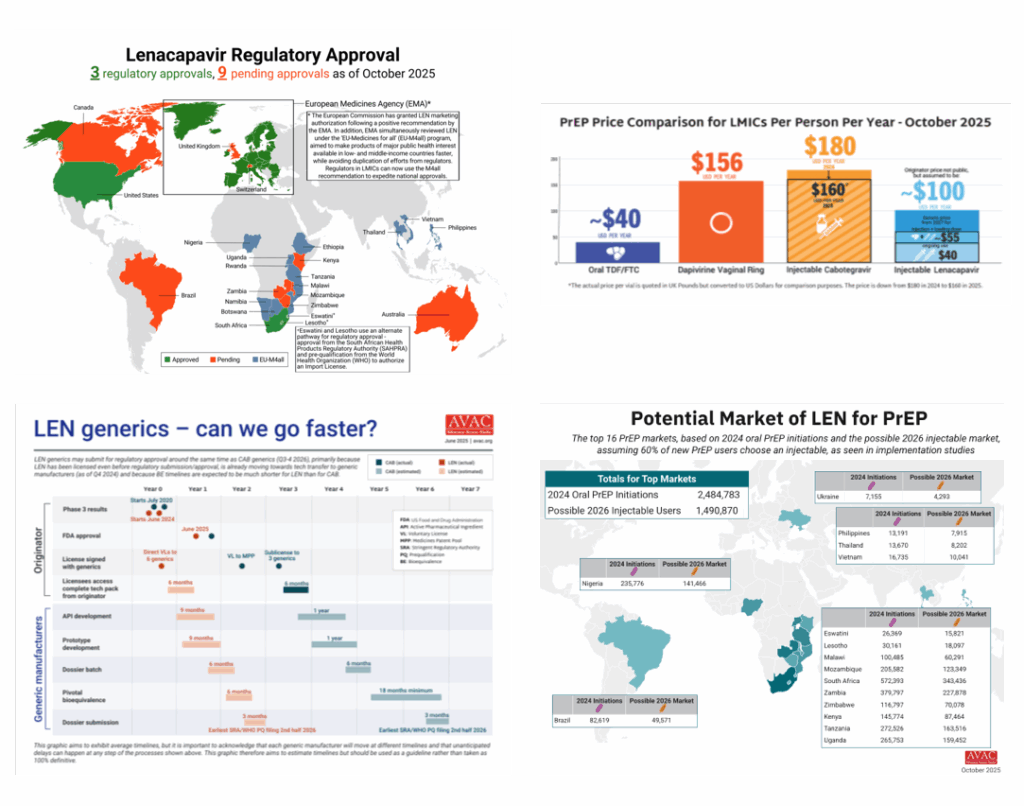
October 27, 2025, brought the latest news on injectable lenacapavir for PrEP (LEN). Just yesterday, South Africa’s regulatory authority approved injectable lenacapvir as PrEP, the first approval in Africa and the fastest Africa approval of PrEP ever. Additional regulatory approvals are pending in Kenya, Malawi, Zambia and Zimbabwe. In addition, earlier in October, the WHO pre-qualified both the oral and injectable forms of LEN using an expedited process. This is progress, but so much more needs to happen to translate this option into a real choice, including: securing price and volume transparency, aligning global funders, and enabling rapid country-level introduction.
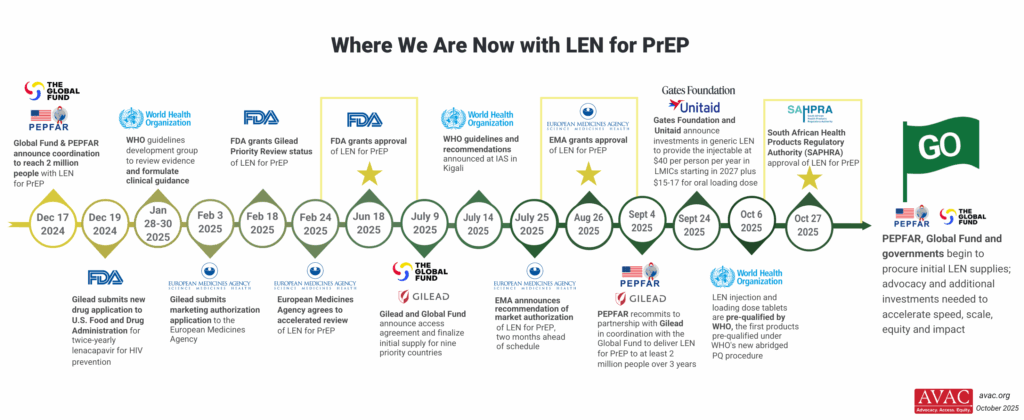
To achieve impact against the epidemic and realize the potential of the full suite of HIV prevention options, policies, planning and targets can and should be bolder, with advocacy and additional investments needed.
Building a sustainable market for LEN—and for PrEP and prevention generally—is essential for affordable and sustainable access and to achieve impact. Demand creation is fundamental to this effort, and so is setting more ambitious goals for total numbers of people on PrEP, which requires upfront investments and coordinated donor support now. Without such action, LEN’s promise could stall, delaying equity and impact. A robust and accelerated rollout will create a foundation for reduced pricing, greater choice, and real momentum in HIV prevention. Our dedicated LEN page on AVAC.org offers additional resources including:
Resources
- Getting PrEP Rollout Right This Time provides key insights from the rollout of oral PrEP and early introduction of injectable cabotegravir (CAB) and the dapivirine vaginal ring (DVR) to inform a faster, smarter and more equitable introduction of future HIV prevention tools, including lenacapavir.
- Gears of Lenacapavir for PrEP Rollout outlines a focused plan for LEN for PrEP rollout over the next few years, specifying priorities by stakeholder and evaluating volume and pricing strategies.
Infographics
Q2: Global health funding is shrinking, but how is the field adapting in this period of disruption?
Global health funding is in steep decline. The Institute for Health Metrics and Evaluation estimates assistance for health dropped by 21% between 2024 and 2025 and will continue dropping over the next five years. In response, the global health field is entering a period of transition, which is marked by smaller pledges from donors, such as Germany’s 1 billion euro commitment to the Global Fund to Fight AIDS, Tuberculosis and Malaria (GF) over the next three years, which is a 25% reduction. There are also increasing calls for consolidation of the fragmented architecture of funding for global health. Leaders at last week’s World Health Summit echoed this urgency, pushing for streamlined agencies and faster reform.
Simultaneously, a new, bold framework for global health sustainability and equity known as The Accra Reset builds upon the public health leadership of African countries to counteract disruptions to the global health governance. It calls for co-creation and mutual accountability, with Global South countries determining agendas and investment in national health systems, data sovereignty, local production, and more in an effort to transform global health as a platform for prosperity rather than a cost.
As global health undergoes a profound transformation, the central task for advocates is to ensure equitable and effective access to care among communities hardest hit by health threats.
Issue Brief

Friends of the Global Fight Against AIDS, Tuberculosis and Malaria (Friends) published an issue brief, How the Global Fund Makes America Stronger and Safer and a call to action for the US to continue its longstanding commitment to pledge $1 for every $2 from other countries.
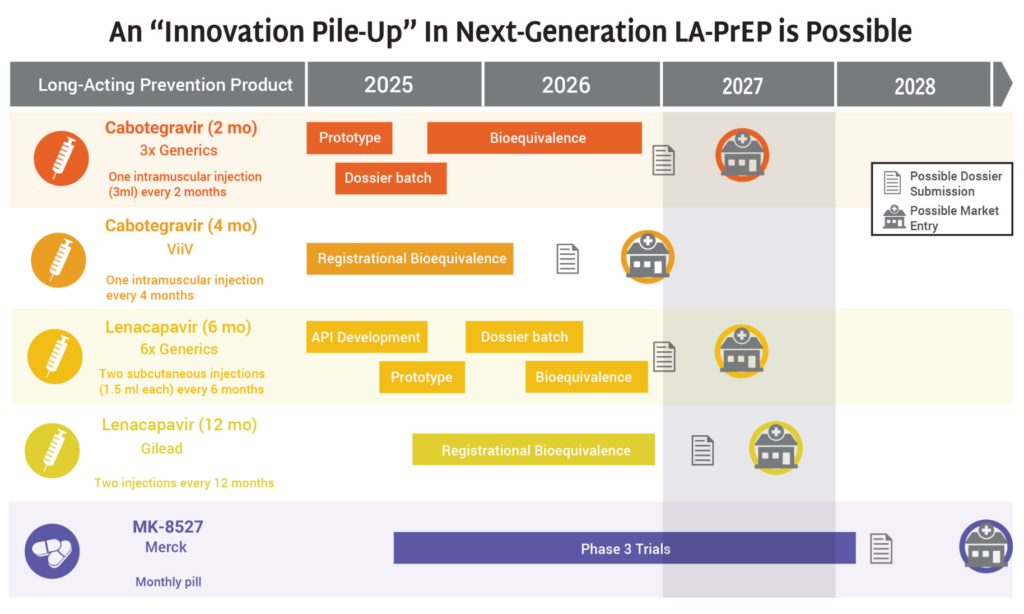
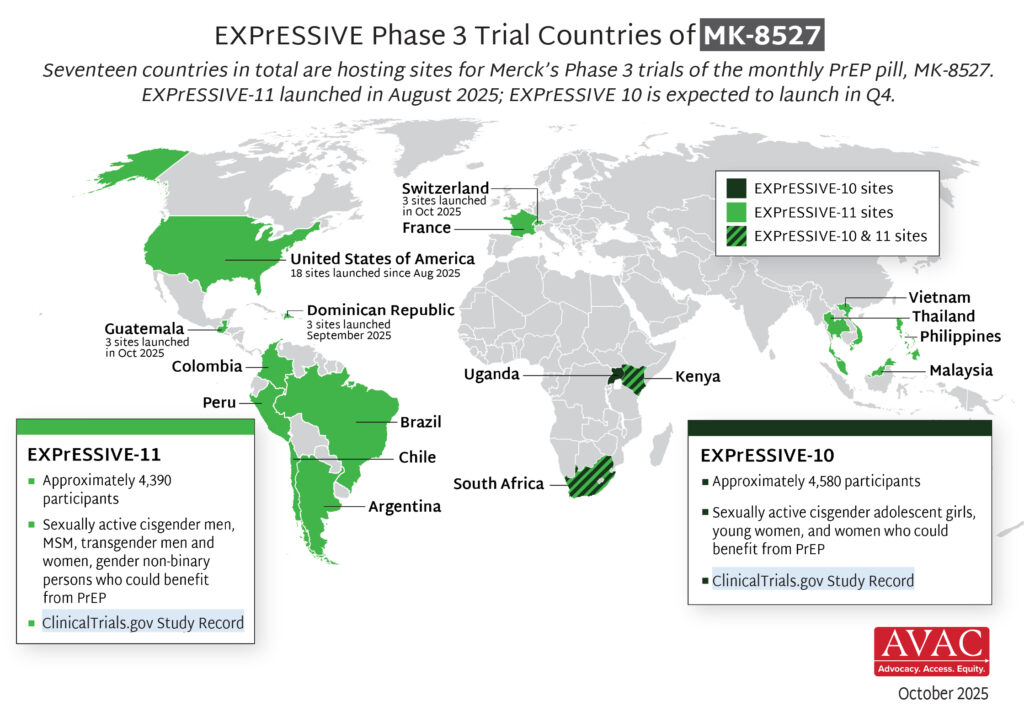
Q3: What’s the status of the R&D pipeline and what action steps are essential now?
From basic science to implementation studies, public support for HIV research has never been more important. An evolving, balanced and innovative pipeline of HIV prevention options is essential to epidemic control and ensuring everyone who needs HIV prevention has access to something that works for them. These resources can inform and support our collective work to defend continued investment in an inclusive and diverse pipeline of HIV prevention research and development (R&D).

These questions are guiding our work at AVAC. We know it will continue to be up to advocates, allies, and global health champions to demand answers, transparency, and programs that deliver sustainable impact. See you in the fight!