This week a $50 billion US foreign affairs spending bill was signed into law, averting severe proposed cuts; a new modelling analysis projects millions of additional preventable deaths by 2030 if global aid cuts continue; and the financial crises facing the UN and WHO continue. We are also following plans to transition or close the Oregon National Primate Research Center, a leading research institution that has contributed enormously in biomedical and HIV research.
US Signs Foreign Affairs Spending Bill Amid Ongoing Uncertainty
The US Congress passed the $50 billion foreign affairs spending bill and the President signed it into law Tuesday, ending a brief government shut-down. The appropriations bill restores billions in foreign assistance, along with companion bills that restore critical support for biomedical research at the NIH and domestic HIV programs, that had been at risk of deep proposed cuts, though it still represents a reduction from previous years and questions remain about how fully the administration will implement the funding and priorities laid out by lawmakers.
IMPLICATIONS: While this bill averts the most severe proposed cuts and sends a strong signal for continued engagement by Congress, the reduced funding level and uncertainty of whether global health and humanitarian programs will receive the funding Congress appropriated leave many reluctant to celebrate. Coordinated advocacy and sustained Congressional oversight will be needed to ensure all appropriated funds are obligated and spent.
READ:
- US Congress passes $50 billion foreign affairs bill—Devex
- UPDATE: Final Passage of FY26 Spending Package Protects Funding for Domestic & Global HIV Programs after Hard Won Fight, Concerns about Funding for DHS Persist—Save HIV Funding
- Devex Newswire: Foreign affairs spending bill becomes law after shutdown—Devex
New Modelling Quantifies the Impact of Aid Cuts
A new modelling analysis published in The Lancet Global Health finds that ongoing cuts to official development assistance, particularly from long-time donors like the US, UK and Germany, could lead to between 9.4 million and 22.6 million additional deaths by 2030 across 93 low- and middle-income countries. This includes more than 5 million children under age five.
IMPLICATIONS: This analysis reinforces the need to sustain strategic investments now to avoid deaths and setbacks on all fronts, from HIV to maternal and child health to chronic diseases.
READ:
- Impact of two decades of humanitarian and development assistance and the projected mortality consequences of current defunding to 2030: retrospective evaluation and forecasting analysis—The Lancet Global Health
- Global aid cuts could lead to 9.4 million deaths by 2030, study projects—Washington Post
- Aid cuts could cause 22m avoidable deaths by 2030, study finds—The Guardian
UN and WHO Face Deepening Financial Crisis
Global health and humanitarian institutions are facing an escalating financial and political crisis. UN Secretary-General António Guterres warned that the United Nations risks “imminent financial collapse” if member states, specifically the US, do not pay their dues on time, or fail to agree to revise the financial rules, which require the UN to repay governments hundreds of millions of dollars in credits for programs, even ones that were never implemented. At the same time, the World Health Organization (WHO) launched its 2026 emergency appeal amid its biggest financial decline in a decade and while the US withdrawals and other countries question their engagement.
IMPLICATIONS: While WHO and the UN pursue reforms toward more sustainable and flexible financing, failure by member states to stabilize funding and modernize governance could strip capacity from global institutions at a moment when they are needed most with major implications for health security, equity, and trust in the global response system.
READ:
- US retreat from WHO harms global health and the HIV response—Positively Aware
- US funding pledge insufficient to avert UN financial woes—Devex
- Global health systems ‘at risk’ as funding cuts bite, warns WHO—UN News
- Building healthy bridges towards peace: WHO launches $1 billion appeal—UN News
- Days After US Leaves WHO, Israel Warns It Faces Pressure To Withdraw—Health Policy Watch
- What kind of leader does WHO need next?—Devex
NIH to Transition Primate Research Center Amid Broader Shift Away from Animal Testing
In the last week, NIH Director, Jay Bhattacharya, confirmed plans to transition the Oregon National Primate Research Center at Oregon Health & Science University (OHSU) into an animal sanctuary. This is the first of possibly seven of the NIH’s National Primate Research Centers to close or transition and is part of a broader national push to reduce animal testing. The NIH said last year it would spend $87 million to develop a standardized alternative to animal testing. OHSU’s board of directors will meet Monday to consider negotiations with the NIH. They previously estimated it would cost $241 million over eight years to close.
IMPLICATIONS: This move reflects a broader pattern of policy decisions that risk eroding the foundational research systems underpinning early-stage biomedical science, including HIV prevention, treatment, and cure research. While developing alternatives to animal research is important, rapidly dismantling animal research—particularly nonhuman primate capacity at these centers and at the CDC—without validated replacements could weaken the early-stage pipeline that has been critical to breakthroughs such as HIV PrEP, PEP, long-acting prevention, vaccine and cure research and development.
READ:
- NIH looks to turn primate research center into a sanctuary—Politico
- As US officials move to reduce animal testing in research, focus may shift to restrictions on imports—STAT
What We’re Reading
- ‘Biblical Diseases’ Could Resurge in Africa, Health Officials Fear—New York Times
- What Will HIV Funding Look Like in 2026?—Bhekisisa
- By-disease investment trends for the first 15 American global health agreements—To End a Plague…Again Substack
- Thanks, but no thanks—The Forsaken Substack
- Gates doubles down on goals in a world weighed down by crisis, CEO says—Devex
- Q&A: How Can Humanitarians Navigate The New Expanded Global Gag Rule?—Health Policy Watch
- Good health is the world’s best investment – and the key to economic resilience—Gavi
- Whose ethics govern global health research?—Nature Medicine
- FDA Commissioner Marty Makary tries to soothe staff concerns over voucher program—STAT
- We asked whether principal investigators have plans in place for how research can continue without them—STAT
- The Only Thing That Will Turn Measles Back—The Atlantic
- Trump Administration to Make It Easier to Fire 50,000 Federal Workers—Wall Street Journal
- Evaluation of an Artificial Intelligence Conversational Chatbot to Enhance HIV Preexposure Prophylaxis Uptake: Development and Usability Internal Testing—Journal of Medical Internet Research
- A Year of Disruption: 5 Resources to Understand Foreign Aid Cuts—Partners in Health
- NIH rolls back red tape on some experiments — spurring excitement and concern—Nature
- Conflicts and Vaccine Hesitancy Undermine Global Immunization Efforts—Health Policy Watch
New Issue of PxWire
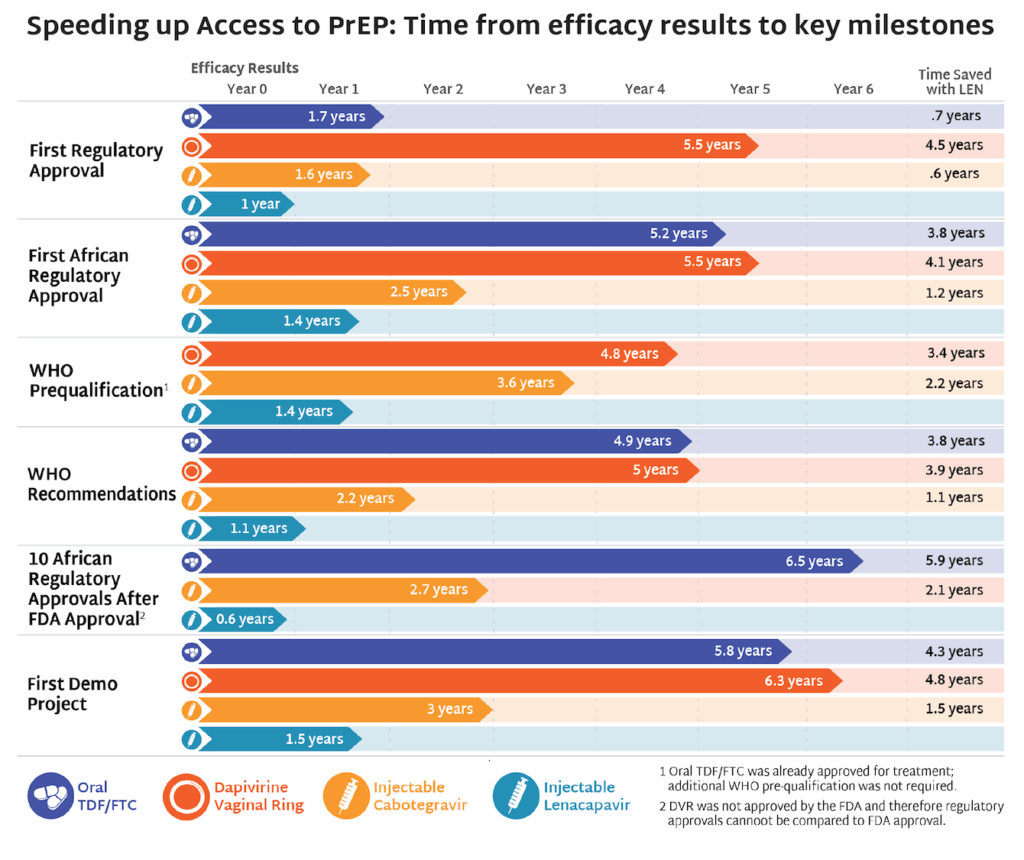
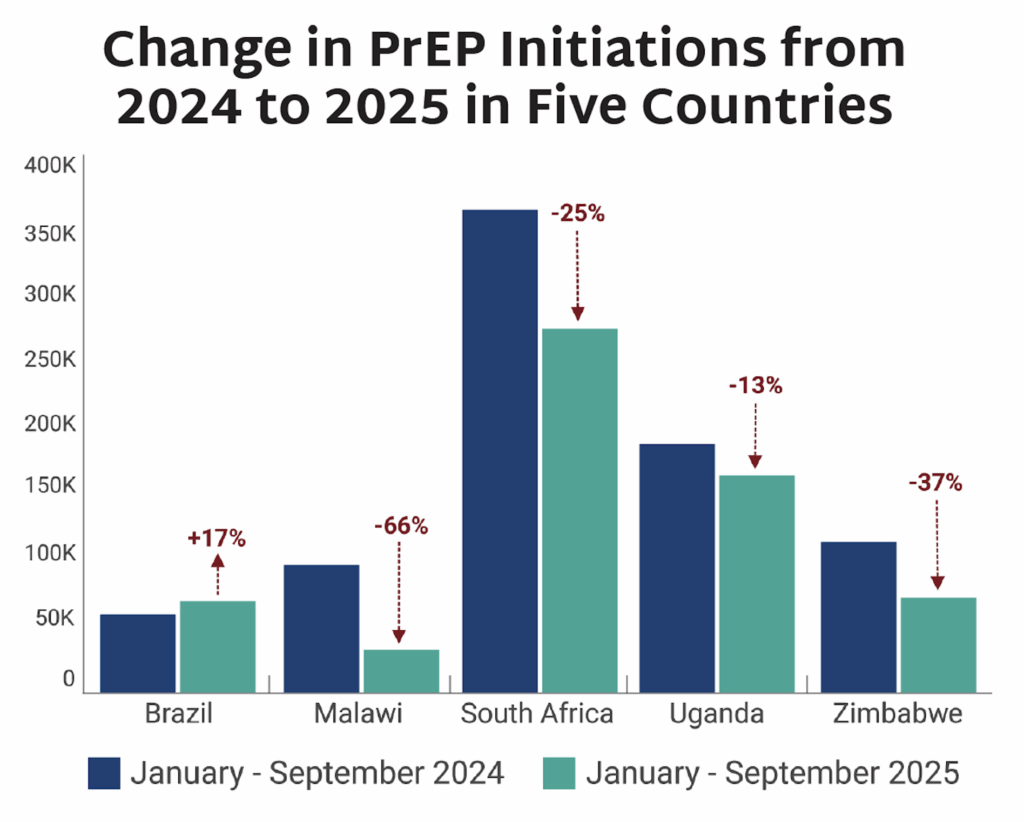
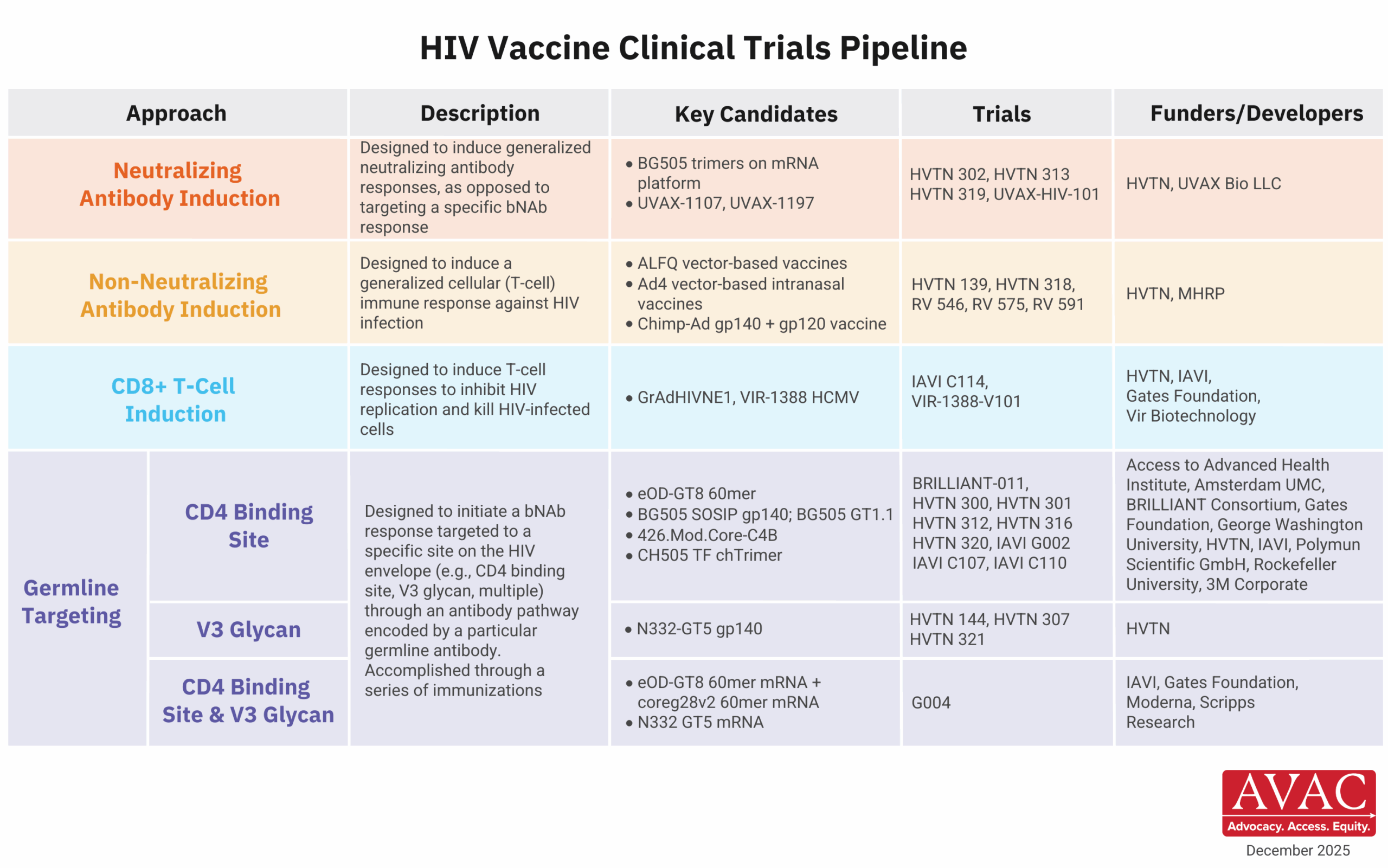
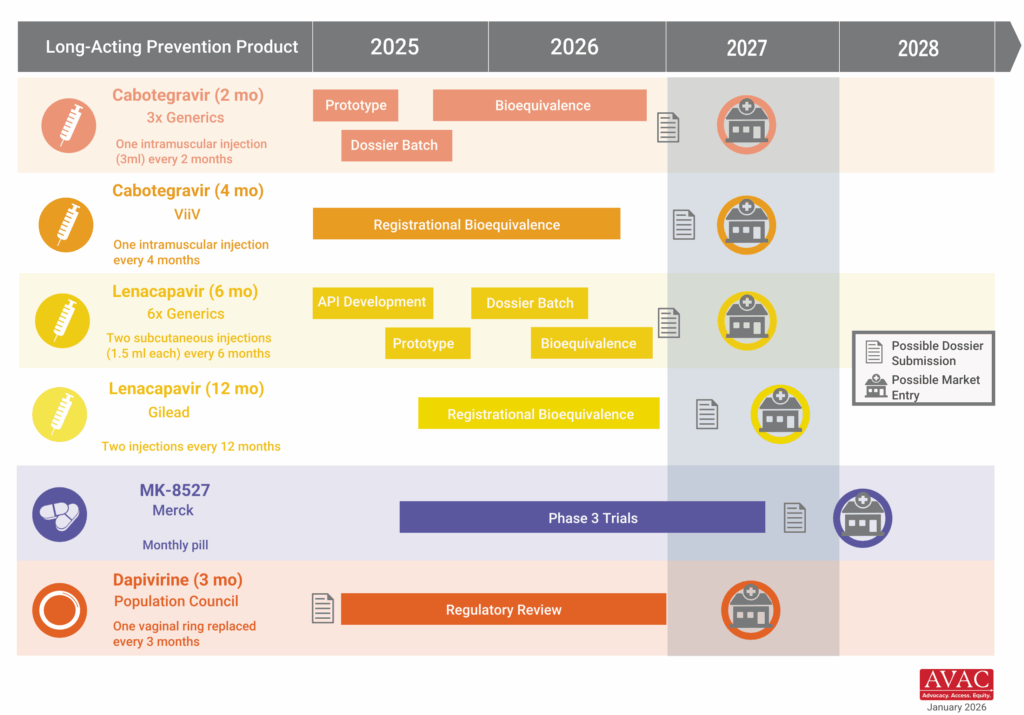
AVAC’s latest issue of PxWire shows reduced initiations of oral PrEP following the US foreign aid freeze; the accelerated rollout of injectable lenacapavir (LEN) for PrEP; and what’s next in the HIV vaccine R&D pipeline.