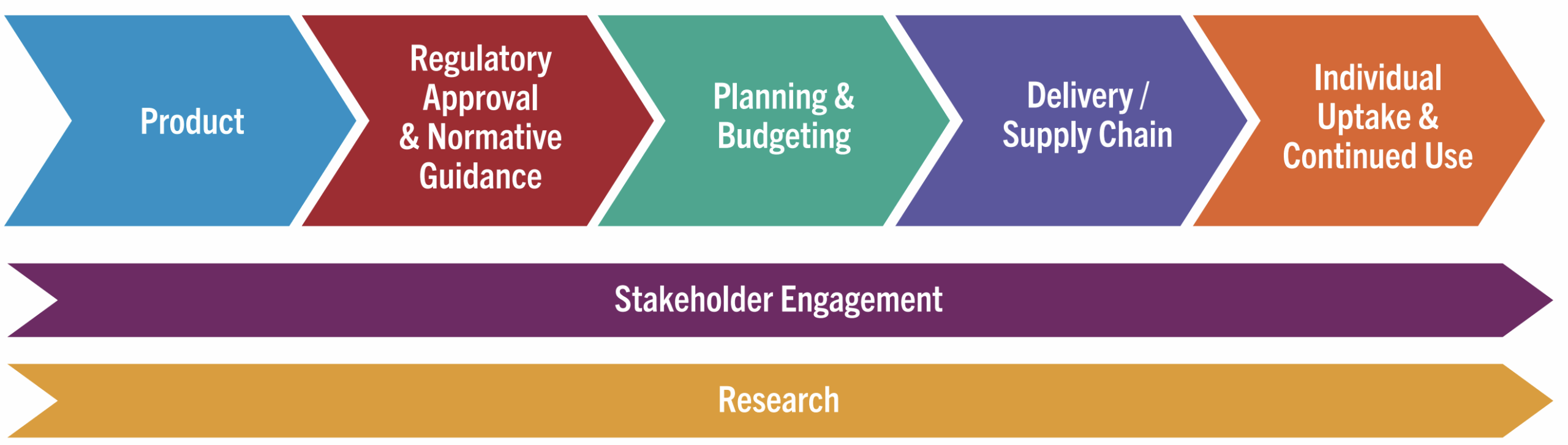
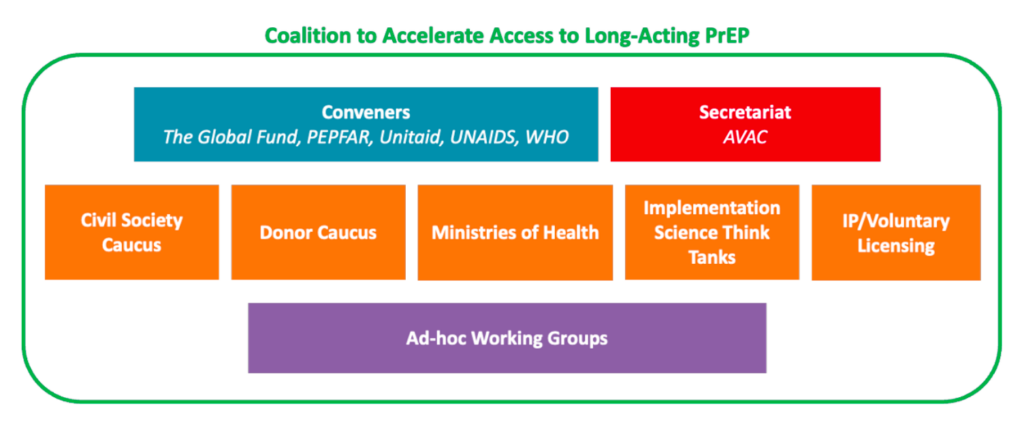
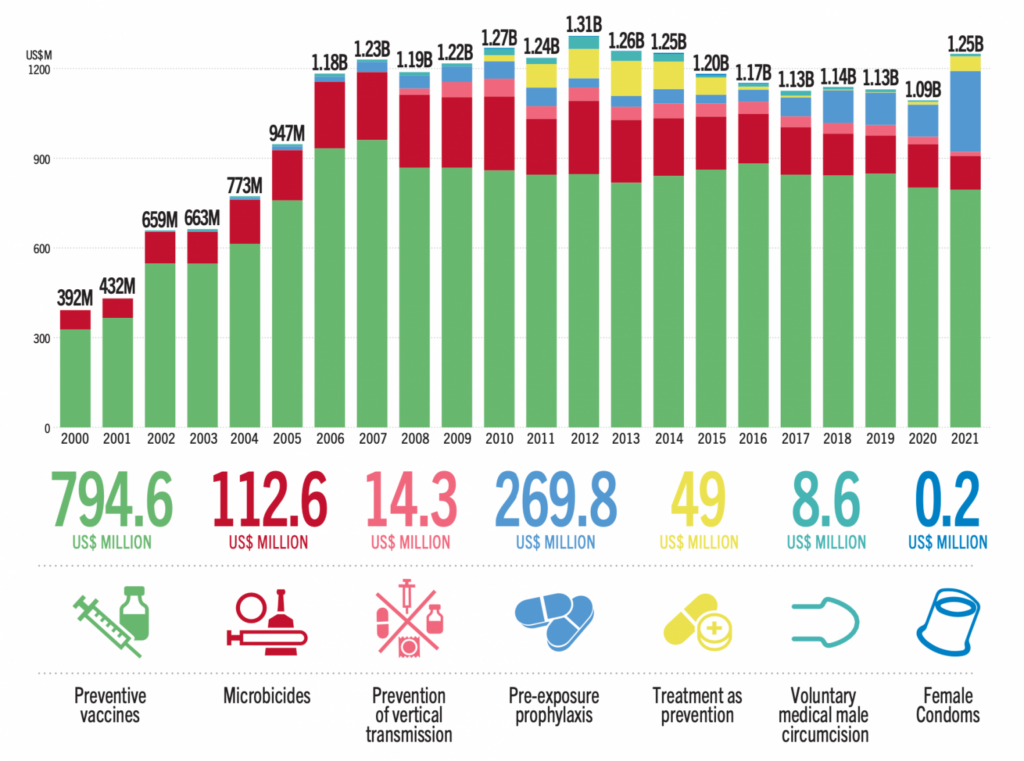
AVAC’s work to advance advocacy for STI prevention and integrate it with HIV prevention and global health equity involves supporting a network of advocates, expanding research literacy, and identifying priorities for the field.
Action That’s Long Overdue
Sexually transmitted infections (STIs) are widespread, remain difficult to prevent, and can have severe health consequences, including increased risk for HIV acquisition. While most STIs are curable, they often go undiagnosed and untreated because they tend to cause few or no initial symptoms. Available diagnostics are often too costly to use for routine screening in many low to middle income countries (LMICs), and to date few vaccines exist to address the most common STIs.
Despite the scope and urgency of the problem, STIs receive little public policy or community attention. These infections are often stigmatizing and their effects are most devastating among groups with little political power, such as adolescent girls and young women, sex workers, gay men, and other men who has sex with men.
STIs intersect with HIV and other key priorities for global health equity- developing an advocacy agenda to expedite research, development and rollout of strategies to test for and prevent STIs that is integrated into a broader response is fundamental to global health. Current knowledge and technology suggest breakthroughs are possible and could deliver better, affordable diagnostics and new prevention options.
Supporting a Network of Advocates
STIs intersect with the concerns of many vibrant advocacy communities including those working in HIV, sexual and reproductive health, cancer, infertility, and maternal and newborn health. AVAC draws from these communities to raise awareness of STI advocacy, and interest and expertise in scientific research on STI vaccines or diagnostics.
Expanding and advancing the pipeline of research and development for diagnostics and vaccines, along with driving policies and funding to bolster use of the diagnostics that already exist, are essential for prevention across global health.
Expanding Research Literacy for STI prevention
AVAC and partners provide a host of resources to track and translate research related to STI prevention.
- STI Watch.org tracks vaccine research for seven of the most common STIs, and features a roadmap for development of new vaccines from WHO, NIH and other partners.
- The Weekly News Digest captures updates from the news on STI R&D.
- AVAC’s STI Clinical Trial Database is updated with STI research on diagnostics and vaccines.
- The STI Advocates Network reaches a growing list of seasoned advocates developing expertise in STI research with news and information for their advocacy. Sign up here.
- STI R&D Investment, AVAC’s resource tracking report provides an annual analysis of global funding trends for STI prevention research.
- Media Science Cafes provides additional resources on STI research for journalists.
- STI subawards provides grantees an opportunity to explore how to better advocate for and communicate about STI vaccines, diagnostics, and other prevention tools. Learn more in the blogpost, Paving the Road for STI Prevention Advocacy.
Identifying Priorities for the Field
A growing network of STI advocates are accelerating the development of a pipeline of new diagnostics and vaccines. This work depends on champions to make the connection between a robust pipeline of STI prevention products, reduced STI prevalence, reduced HIV risk, and dramatic strides in the improved health of women and other key populations.
With ready access to STI-focused data and information provided by the resources above, advocates are identifying baseline data, gaps and resource needs; and consolidating messages and advocacy priorities. This in turn informs collaborations with health and political leaders to set goals for research, investment, policy, planning, and integrated programs for STI and HIV prevention.