Resources
AVAC’s Resource Database contains educational and advocacy materials covering a wide range of issues on biomedical prevention of HIV, STIs, COVID-19 and emerging health threats—from research to rollout.
To search for clinical trials and detailed information on products in development, visit our Prevention Research & Development Database

Results
showing 1-10 of 243
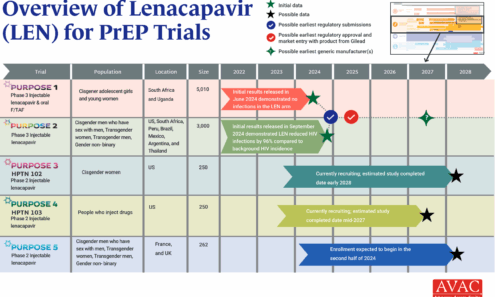
An Overview of Lenacapavir for PrEP Trials
The PURPOSE trials evaluate the safety and efficacy of injectable lenacapavir (LEN), an investigational antiretroviral (ARV) drug being studied as a potential PrEP product. This graphic shows the latest status of all five trials including the groundbreaking results of PURPOSE 1 and PURPOSE 2.
Prevention Option:
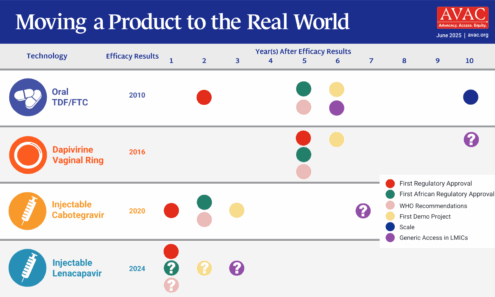
Moving a Product to the Real World
To reach the UNAIDS target of 10 million PrEP users by 2025, initiations of oral PrEP alone will not be enough—and this graphic shows that the field is beginning to apply past lessons to accelerate introduction of injectable cabotegravir.
Prevention Option:
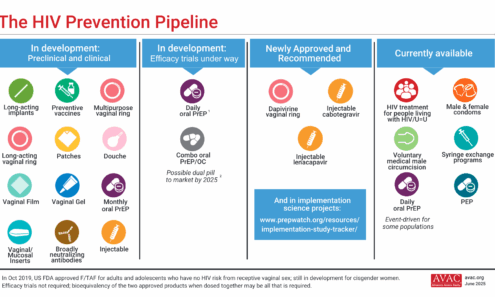
The HIV Prevention Pipeline
This graphic shows currently available options for HIV prevention, newly approved and recommended treatment, and those in development.
Prevention Option:
The Scientific Journey of Lenacapavir — and the Urgency to Defend HIV Prevention Science
On June 11, AVAC hosted a conversation, The Scientific Journey of Lenacapavir: From basic science to clinical development to impact, to explore how US support from NIH for basic science and South Africa’s clinical research infrastructure made possible the development of lenacapavir for PrEP (LEN), a discovery in HIV prevention that went on to be named Science magazine’s 2024 Breakthrough of the Year.
Prevention Option:
HIV Prevention R&D at Risk
AVAC’s analysis of the impact of US Government funding cuts, terminated projects, and other policy changes on the HIV prevention research and development (R&D) pipeline, and on HIV research broadly.

FAPP Response to NIH Cuts to Indirect Research Costs
Federal AIDS Policy Partnership Research Working Group urges Congressional leaders to support the restraining order preventing the National Institutes of Health (NIH) from unlawfully stripping funds that sustain cutting-edge medical and public health research at universities and research institutions nationwide.
AVAC’s Most Downloaded Resources of 2024
From the implementation of DoxyPEP to the game-changing trial results of lenacapavir for PrEP, 2024 has been a landmark year for advancements in HIV and STI prevention. AVAC’s most downloaded resources capture these pivotal milestones, offering essential insights and tools to power your advocacy. Dive into the highlights and stay informed about the strategies shaping the future of HIV prevention.
An Advocate’s Guide to Research in Pregnant and Lactating Populations
A resource that provides background on the need for research in pregnant and lactating populations and how advocates can advance inclusion.

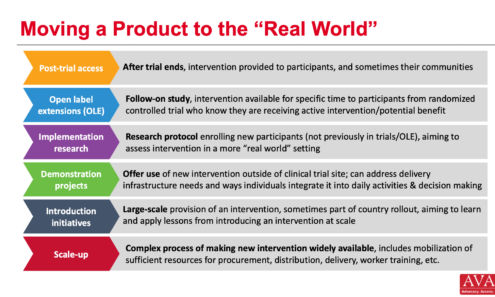
Moving a Product to the “Real World”
A visual roadmap illustrating the key stages transitioning a product from clinical trials to large-scale real-world implementation.
Prevention Option:
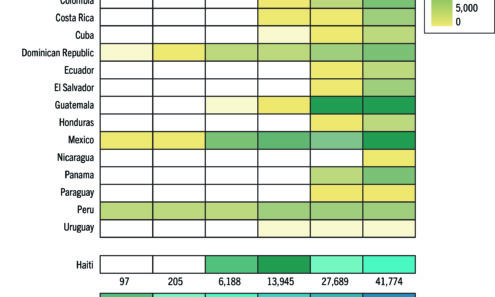
PrEP Initiations in Latin America, 2019-2024
Latin American countries account for 306,000 cumulative PrEP initiations in the Global PrEP Tracker as of Q2 2024, which represent 4% of the global total. Since 2019, PrEP initiations across the region continue to increase at a steady rate, with...
Prevention Option:
showing 1-10 of 243