This timeline shows the potential time points when the next-generation of HIV prevention options might find their way into new programs.
Years Ahead in HIV Prevention Research: Time to Market
People’s Research Agenda
The People’s Research Agenda sets out a people-centered framework for equitable and accelerated R&D and product introduction. It tracks the science, shows where investments align—or fail to align—with community-defined priorities, and spotlights critical gaps in the pipeline of prevention options needed to meet the diverse realities of all populations.
At A Glance: The MPT R&D Pipeline
This graphic shows the status of products in development.
Spotlight on MPTs Addressing STIs
This graphic outlines the development journey of multipurpose technologies (MPTs) that guard against STIs, including HIV, while also preventing pregnancy. It tracks the advancement of various potential products through different trial stages, emphasizing their combined protective roles.
Avac Event
Introducing the Dual Prevention Pill: Lessons Learned and What’s Next for Regulatory, Research, and Rollout
This webinar has been cancelled because funding was pulled by the new US administration. Follow critical developments in US policies and their impact on global health via our new newsletter. Learn more here.
Join the IMPT and guest speakers from AVAC and Population Council for a discussion on the dual prevention pill (DPP)—a single pill that combines oral pre-exposure prophylaxis (PrEP) and oral contraception (OC) to prevent HIV and pregnancy. If approved, the DPP will be the first multi-purpose prevention technology (MPT) to be marketed since condoms.
The discussion will include real-time learnings to inform the broader MPT field on the DPP’s regulatory approval process, acceptability study results in South Africa and Zimbabwe, implementation updates, and lessons learned.
There will be a Q&A session following the presentations.
AVAC’s Guide to HIVR4P 2024 in Lima
We are looking ahead to the biennial HIV Research for Prevention 2024 conference in Lima, Peru next week, 6-10 October. HIVR4P is a space where biomedical HIV prevention research, policy and programs takes center stage. Whether you’ll be in Lima or are following from afar, AVAC will keep you connected!
Read on for information on AVAC sessions, a sortable roadmap, the Advocates’ Corner (open all week) and more!

Resources
- Use AVAC’s Prevention Roadmap of conference sessions and satellites to find what interests you the most. You can download it as a sortable spreadsheet or PDF.
- Advocates’ Corner: If you plan to be in Lima, be sure to join us and our CASPR partners at the Advocates’ Corner to take the conversations and themes deeper. The Advocates’ Corner will be open throughout the conference hosting a program of activities along with materials displays and opportunities for informal networking. Be sure to check the events page for updates on programming.
- AVAC’s Coverage: From the latest news on injectable lenacapavir, to updates on the development of next generation prevention options, to the complex work of implementing the tools that exist today and all the advocacy needed to get it all done, our email dispatches to the Advocates’ Network keep you informed. Follow events in real time on Twitter at #HIVR4P2024 and Instagram.
- People’s Research Agenda: During HIVR4P, we’ll be releasing the new People’s Research Agenda, a global initiative driven by communities and advocates to define the most urgent priorities, research questions and recommendations for HIV prevention research. We hope it serves as a guide to what is – and should be – discussed at HIVR4P and beyond.

Satellites and Sessions Featuring AVAC and Partners
Sunday, 6 October
- Satellite: Creating a Menu of Options: Early R&D of HIV prevention products for women, the MATRIX way, 08:30 – 10:00
- Satellite: The Continued Relevance of HIV Vaccines in the Age of Long-Acting Antiretrovirals: Insights from India and Sub-Saharan Africa, 10:30 – 12:00
- Satellite: Enhancing service providers’ engagement in PrEP delivery, uptake, and retention in Latin America & the Caribbean, 10:30 – 11:30
- Satellite: The brightest under 30: Celebrating youth voices and promoting meaningful youth engagement in HIV prevention research, 10:30 – 12:00
- Satellite: Catalyzing Progress in the Inclusion of Pregnant and Lactating People in HIV Prevention Research, 12:30 – 14:00
- Satellite: Delivering on the promise: Defining optimal implementation strategies and service delivery packages for the Dual Prevention Pill,14:30 – 16:00
- Satellite: What’s Really Going to Work in the Lives of AGYW? Innovations in Acceptability and Mobile Health Support Interventions for HIV Prevention, 12:30 – 14:00
- Satellite: Understanding the role and power of advocates and researchers in advancing Discovery Medicine Vaccine Trials (DMVTs) and the development of Broadly Neutralizing Antibodies (bnAbs) for HIV Prevention, 16:30 – 17:30
Monday, 7 October
- Satellite: Leveraging SBR to Engage and Empower communities in HIV Prevention Research, 09:00 – 10:00
- Satellite: Manifest Choice: Enabling a future free of HIV, 10:30 – 11:30
- Satellite: Advancing Good Participatory Practices (GPP) in Research: Enhancing Community Engagement for Impact, 12:30 – 13:30
Tuesday, 8 October
- Oral abstract: The big picture: Global trends in HIV prevention, 11:00 – 12:30
AVAC’s Catherine Verde Hashim will present the abstract, Identifying global typologies of HIV PrEP implementation: an analysis of global data using PrEP-to-need ratios and PrEP distribution volumes. - Oral abstract: Novel antiretrovirals and formulations for prevention, 11:00 – 12:40
Jim Pickett will co-moderate this session which will discuss new data on islatravir and lenacapavir for PrEP, U=U and more.
- Symposium: Quo vadis: Future design and conduct of vaccine and bNAb clinical trials, 13:30 – 15:00
AVAC’s Grace Kumwenda and colleagues will discuss the viability and practicality of bNAbs as HIV prevention tools.
- Symposium: Prevention product profiles for future options, including long-acting PrEP formulations and products, 13:30 – 15:00
Moderated by Mitchell Warren, this session will discuss the issues of choice and combination products in HIV prevention, and will look at Target Product Profiles for various technologies, especially long-acting PrEP options.
Wednesday, 9 October
- Symposium: Reducing burdens and barriers to expand the use of HIV prevention options, 13:30 – 15:00
This session will explore the promise, potential and risks of using remote tools, such as telemedicine, virtual tools, apps and self-testing and the impact of other tools used to expand access and uptake of HIV prevention modalities. It will also review approaches to overcome misinformation and mistrust.
Thursday, 10 October
- Oral abstract: Policy and legal barriers to HIV services, 08:30 – 10:00
Brian Minalga of Fred Hutchinson Cancer Center will present, The transgender scorecard: ensuring representation in HIV prevention research.
- Oral abstract: Driving PrEP implementation through community engaged science, 13:00 – 14:30
Esther Nakkazi will present, Using local languages for accurate science reporting in Media Science Cafés in East and Southern Africa.
Find these resources, conference highlights and more at AVAC’s dedicated HIVR4P 2024 page. And watch this space for new opportunities to come together and shape what happens next.
Avac Event
HIVR4P 2024
The 5th HIV Research for Prevention (R4P) conference is being held in Lima, Peru from 6 to 10 October. Held every two years, HIVR4P is the only global conference to focused exclusively on biomedical HIV prevention, including AIDS vaccines, microbicides, PrEP, treatment as prevention and other approaches.
See below for conference highlights, recaps and announcements.
Conference Highlights and Recaps
- Pre-conference Highlights from HIVR4P 2024
- HIVR4P Highlights Access and Choice: Inextricably Linked
- HIVR4P Wednesday Highlights: Access Access Access
- HIVR4P Thursday Highlights: Centering Communities
Announcements


Advocates’ Guide to Multipurpose Prevention Technologies (MPTs)
Multipurpose prevention technologies (MPTs) are products designed to simultaneously address more than one sexual and reproductive health concern. This advocates’ guide shows the pipeline of products in development, discusses why MPTs are needed, investment, and what advocates can do to push for MPT development and introduction.
SRH + HIV integration advocacy, Pandemic Accord, GPP and more!
AVAC’s round-up of resources, updates and insights this week includes a new roadmap for sexual and reproductive health (SRH) and HIV integration, resources to support an equitable Pandemic Accord, innovations in Good Participatory Practices (GPP) and more!
The power of choice in contraception, sexual health and HIV prevention this World Contraception Day
Roadmap: Sexual and Reproductive Health (SRH) Integration Roadmap

Copper Rose Zambia, as a part of CASPR and AVAC launched a new resource addressing the critical need for integrated SRH and HIV services. This roadmap provides key steps for success, focusing on collaboration, strategic mapping and targeted advocacy.
Advocate’s Guide: Advocates’ Guide to Multipurpose Prevention Technologies (MPTs)
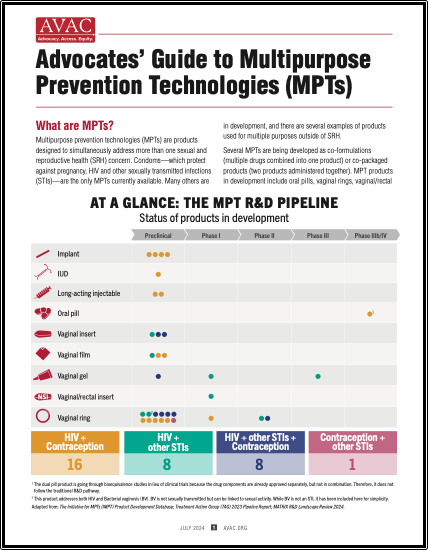
MPTs are products designed to simultaneously address more than one sexual and reproductive health concern. This advocates’ guide shows the pipeline of products in development, discusses why MPTs are needed, investment, and what advocates can do to push for MPT development and introduction.
What will it take for an equitable Pandemic Accord?
Call to Action: Pandemic Accord Priorities from the Coalition of Advocates for Global Health and Pandemic Preparedness

A group of organizations advocating for an integrated and holistic approach to preparedness that emphasizes equity, inclusion, and synergies of multiple global health programs in advancing preparedness, shares five priorities in Pandemic Accord negotiations.
UNGA Side Event: Restrategizing Civil Society Engagement for Pandemic and Global Governance

AVAC’s Sam Rick moderated CISDI’s event alongside Nina Schwalbe, Lawrence Gostin, Eloise Todd and others, reminding the audience that for pandemic prevention, preparedness and response (PPPR) to succeed, lessons from the HIV response must be integrated into the architecture being built for PPPR.
Good Participatory Practices in action
Call for Applications: Now Accepting Applications for the 2024 Good Participatory Practice Online Course
The 2024 Good Participatory Practice Online Course is now accepting applications for 30 spots! This course offering will run 14 October – 20 December 2024. The application deadline is 9 October.
Recording: Innovations in GPP

Recording / Clever Chilende Slides / Sarah Read Slides / Ntando Yola Slides
What’s Next for the Dual Prevention Pill (DPP)
Dear Advocate,
The latest edition of the Journal of the International AIDS Society features newly published research by AVAC and partners on the benefits of delivering family planning and PrEP using pharmacies, e-pharmacies and telemedicine, in addition to private sector clinics. The article, Harnessing private sector strategies for family planning to deliver the Dual Prevention Pill, the first multipurpose prevention technology with pre-exposure prophylaxis, in an expanding HIV prevention landscape, demonstrates why these delivery methods should be prioritized for rolling out the Dual Prevention Pill (DPP), a daily pill that combines oral PrEP with an oral contraceptive to prevent both unintended pregnancy and HIV.

The DPP is the Multipurpose Prevention Technology (MPT) closest to market and the first-ever with PrEP. Following the recent successful conclusion of a bioequivalence study, where researchers demonstrated the active ingredients functioned in the body the same as when PrEP and oral contraception are taken separately, the DPP could be approved by regulators by late 2025. The DPP could be a desirable choice for women seeking an option that will meet multiple needs in their sexual and reproductive health (SRH).
The research found that a significant proportion of family planning (FP) services in Kenya (22%), South Africa (11.4%) and Zimbabwe (17.3%) are delivered using the private sector (such as private provider networks, pharmacies, e-pharmacies and telemedicine). But these channels remain underutilized and represent a largely untapped — yet growing — delivery channel with great potential to expand access to PrEP.
“Addressing the underlying reasons why this is the case will be a prerequisite to DPP rollout, not just in these three countries, but in all countries with high HIV incidence where the private sector is a popular source for FP, such as Eswatini, Lesotho, Malawi, Namibia, Uganda and Zambia”.
The authors write.
Based on these findings, regulators should update national guidelines to allow for more diverse PrEP delivery. Training on PrEP delivery could be expanded among nurses and other providers, as well as doctors. Clinics, pharmacies, telemedecine and e-pharmacies could offer PrEP. Implementers and researchers should also undertake research to better understand willingness and ability to pay, how these factors align with the cost of DPP delivery, and what additional subsidy may be needed to ensure successful rollout of the DPP.
This research supports a Market Preparation and Introduction Strategy that is guiding plans for how, where and to whom the DPP is introduced. Providing users a range of options to access the DPP in non-traditional channels will minimize stigma, improve convenience, and offer discretion — all of which are features that will increase overall uptake and continuation. See our resources on the DPP below.
- The Dual Prevention Pill: Market Preparation and Introduction Strategy, informs priorities and planning for DPP rollout. See the Strategy Executive Summary here.
- Equipping Providers To Offer Novel MPTs: Developing Counseling Messages for the Dual Prevention Pill in Clinical Studies and Beyond
- Cost-Effectiveness of the Dual Prevention Pill for Contraception and HIV Pre-Exposure Prophylaxis
- How Might We Motivate Uptake of the Dual Prevention Pill? Findings From Human-Centered Design Research with Potential End Users, Male Partners, and Healthcare Providers