This timeline shows the potential time points when the next-generation of HIV prevention options might find their way into new programs.
This file is also available to download as a PowerPoint file.
This timeline shows the potential time points when the next-generation of HIV prevention options might find their way into new programs.
This file is also available to download as a PowerPoint file.
Avac Event
This webinar has been cancelled because funding was pulled by the new US administration. Follow critical developments in US policies and their impact on global health via our new newsletter. Learn more here.
Join the IMPT and guest speakers from AVAC and Population Council for a discussion on the dual prevention pill (DPP)—a single pill that combines oral pre-exposure prophylaxis (PrEP) and oral contraception (OC) to prevent HIV and pregnancy. If approved, the DPP will be the first multi-purpose prevention technology (MPT) to be marketed since condoms.
The discussion will include real-time learnings to inform the broader MPT field on the DPP’s regulatory approval process, acceptability study results in South Africa and Zimbabwe, implementation updates, and lessons learned.
There will be a Q&A session following the presentations.
This graphic outlines the development journey of multipurpose technologies (MPTs) that guard against STIs, including HIV, while also preventing pregnancy. It tracks the advancement of various potential products through different trial stages, emphasizing their combined protective roles. Excerpted from our Advocates’ Guide to Multipurpose Technologies.
We are looking ahead to the biennial HIV Research for Prevention 2024 conference in Lima, Peru next week, 6-10 October. HIVR4P is a space where biomedical HIV prevention research, policy and programs takes center stage. Whether you’ll be in Lima or are following from afar, AVAC will keep you connected!
Read on for information on AVAC sessions, a sortable roadmap, the Advocates’ Corner (open all week) and more!


Sunday, 6 October
Monday, 7 October
Tuesday, 8 October
Wednesday, 9 October
Thursday, 10 October
Find these resources, conference highlights and more at AVAC’s dedicated HIVR4P 2024 page. And watch this space for new opportunities to come together and shape what happens next.
Avac Event
The 5th HIV Research for Prevention (R4P) conference is being held in Lima, Peru from 6 to 10 October. Held every two years, HIVR4P is the only global conference to focused exclusively on biomedical HIV prevention, including AIDS vaccines, microbicides, PrEP, treatment as prevention and other approaches.
See below for conference highlights, recaps and announcements.


This graphic shows the status of products in development. Excerpted from our Advocates’ Guide to Multipurpose Technologies.
Multipurpose prevention technologies (MPTs) are products designed to simultaneously address more than one sexual and reproductive health concern. This advocates’ guide shows the pipeline of products in development, discusses why MPTs are needed, investment, and what advocates can do to push for MPT development and introduction.
AVAC’s round-up of resources, updates and insights this week includes a new roadmap for sexual and reproductive health (SRH) and HIV integration, resources to support an equitable Pandemic Accord, innovations in Good Participatory Practices (GPP) and more!
Roadmap: Sexual and Reproductive Health (SRH) Integration Roadmap

Copper Rose Zambia, as a part of CASPR and AVAC launched a new resource addressing the critical need for integrated SRH and HIV services. This roadmap provides key steps for success, focusing on collaboration, strategic mapping and targeted advocacy.
Advocate’s Guide: Advocates’ Guide to Multipurpose Prevention Technologies (MPTs)
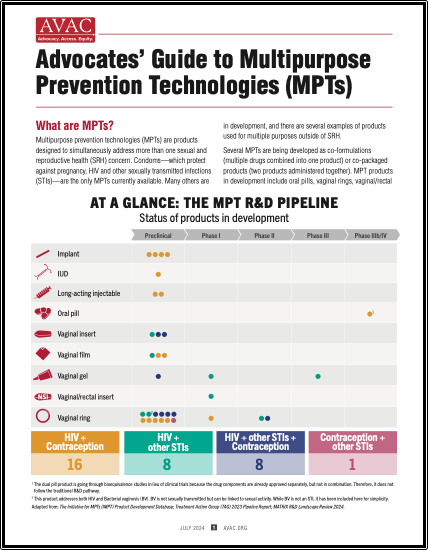
MPTs are products designed to simultaneously address more than one sexual and reproductive health concern. This advocates’ guide shows the pipeline of products in development, discusses why MPTs are needed, investment, and what advocates can do to push for MPT development and introduction.
Call to Action: Pandemic Accord Priorities from the Coalition of Advocates for Global Health and Pandemic Preparedness

A group of organizations advocating for an integrated and holistic approach to preparedness that emphasizes equity, inclusion, and synergies of multiple global health programs in advancing preparedness, shares five priorities in Pandemic Accord negotiations.
UNGA Side Event: Restrategizing Civil Society Engagement for Pandemic and Global Governance

AVAC’s Sam Rick moderated CISDI’s event alongside Nina Schwalbe, Lawrence Gostin, Eloise Todd and others, reminding the audience that for pandemic prevention, preparedness and response (PPPR) to succeed, lessons from the HIV response must be integrated into the architecture being built for PPPR.
Call for Applications: Now Accepting Applications for the 2024 Good Participatory Practice Online Course
The 2024 Good Participatory Practice Online Course is now accepting applications for 30 spots! This course offering will run 14 October – 20 December 2024. The application deadline is 9 October.
Recording: Innovations in GPP

Recording / Clever Chilende Slides / Sarah Read Slides / Ntando Yola Slides
Dear Advocate,
The latest edition of the Journal of the International AIDS Society features newly published research by AVAC and partners on the benefits of delivering family planning and PrEP using pharmacies, e-pharmacies and telemedicine, in addition to private sector clinics. The article, Harnessing private sector strategies for family planning to deliver the Dual Prevention Pill, the first multipurpose prevention technology with pre-exposure prophylaxis, in an expanding HIV prevention landscape, demonstrates why these delivery methods should be prioritized for rolling out the Dual Prevention Pill (DPP), a daily pill that combines oral PrEP with an oral contraceptive to prevent both unintended pregnancy and HIV.

The DPP is the Multipurpose Prevention Technology (MPT) closest to market and the first-ever with PrEP. Following the recent successful conclusion of a bioequivalence study, where researchers demonstrated the active ingredients functioned in the body the same as when PrEP and oral contraception are taken separately, the DPP could be approved by regulators by late 2025. The DPP could be a desirable choice for women seeking an option that will meet multiple needs in their sexual and reproductive health (SRH).
The research found that a significant proportion of family planning (FP) services in Kenya (22%), South Africa (11.4%) and Zimbabwe (17.3%) are delivered using the private sector (such as private provider networks, pharmacies, e-pharmacies and telemedicine). But these channels remain underutilized and represent a largely untapped — yet growing — delivery channel with great potential to expand access to PrEP.
“Addressing the underlying reasons why this is the case will be a prerequisite to DPP rollout, not just in these three countries, but in all countries with high HIV incidence where the private sector is a popular source for FP, such as Eswatini, Lesotho, Malawi, Namibia, Uganda and Zambia”.
The authors write.
Based on these findings, regulators should update national guidelines to allow for more diverse PrEP delivery. Training on PrEP delivery could be expanded among nurses and other providers, as well as doctors. Clinics, pharmacies, telemedecine and e-pharmacies could offer PrEP. Implementers and researchers should also undertake research to better understand willingness and ability to pay, how these factors align with the cost of DPP delivery, and what additional subsidy may be needed to ensure successful rollout of the DPP.
This research supports a Market Preparation and Introduction Strategy that is guiding plans for how, where and to whom the DPP is introduced. Providing users a range of options to access the DPP in non-traditional channels will minimize stigma, improve convenience, and offer discretion — all of which are features that will increase overall uptake and continuation. See our resources on the DPP below.
Working with colleagues, AVACers Cat Verde Hashim and Kate Segal published this research article in the Journal of the International AIDS Society. The researchers undertook qualitative research in Kenya, South Africa and Zimbabwe to prioritize private sector service delivery approaches for the introduction of the DPP.