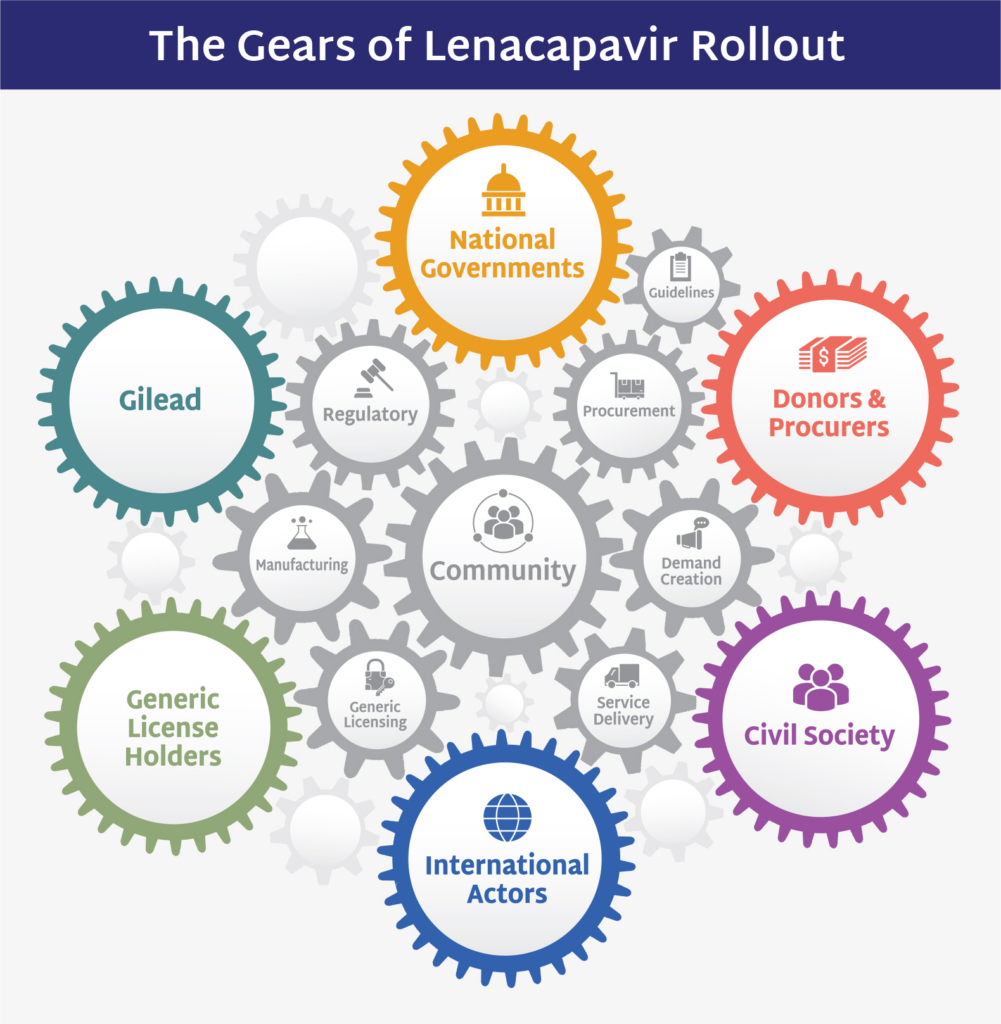
As we enter a new year, we also enter the midway point of the AVAC 2024-2025 Fellowship program, which runs 18 months. Fellows and their projects are taking giant strides in new areas of advocacy, and realizing strategic wins toward epidemic-bending goals, with much more to come! For more than a decade, Fellows have tackled critical issues for the field, focusing on U=U and new technologies, including long-acting PrEP, the Dual Prevention Pill (DPP) and the dapivirine ring (DVR). 2024-2025 fellows have added new areas of focus including on Pandemic Prevention, Preparedness and Response (PPPR); anal health; and HIV prevention in prisons.

Here are some of their accomplishments of the 2024–2025 Fellows Program
Ezra Meme (Uganda) is AVAC’s first Fellow to advance a Pandemic Prevention, Preparedness and Response (PPPR) agenda. He contributed to the development of the Uganda National Action Plan for Health and Security to bolster public health emergency centers throughout the country. While the billions committed in Ugandan shillings still need to be secured, Ezra will continue to monitor and advocate for this unprecedented public health project. He also successfully advocated for the government’s launch of the Antimicrobial National Action plan. And, he’s been closely monitoring Mpox, advocating for a digital mapping tool to track its control efforts.
Bahati Thomas Haule (Tanzania) is laser focused on scaling up and normalizing U=U and the accompanying need for timely viral load testing and results. She’s gotten buy-in from PEPFAR and UNAIDS to support a substantial U=U media campaign and she’s collaborating with the Elizabeth Glaser Pediatric AIDS Foundation to spread knowledge of their model U=U program for “nationwide utilization.” She’s also in dialogue with Global Fund to support increased lab capacity and provider training. Bahati presented to the UK Parliament on the need to support LEN for PrEP and for World AIDS Day, she published an opinion piece in the Swahili newspaper Mwananchi, advocating for ARV-based prevention.
Mokone Rantsoelaba (Lesotho) is AVAC’s first Fellow to advocate for HIV services in prison. After visiting nearly all his country’s correctional facilities, he released an assessment of HIV services for the incarcerated in Lesotho. His work was so well received that he was asked to integrate many of his recommendations into Lesotho’s Correctional Services Healthcare Policy, including the training of providers and fast-tracking all new prevention methods for scale-up in prisons. Mokone initiated and continues to convene the Southern African Development Community (SADC) regional correctional institutions along with the United Nations Office on Drugs and Crime (UNODC) to share best practices. Mokone has also been invited as a guest columnist for one of Lesotho’s top publications.
Sammy Anyula Gorigo (Kenya) is AVAC’s first Fellow to spotlight anal health. Specifically, Sammy’s been promoting HPV vaccines, screening and treatment for all men, particularly gay and bisexual men and other MSM. Thus far, he integrated HPV screening as a standard of practice into Nairobi County Health Management. He updated two separate guidelines to include anal health care for men—Kenya’s Standard Operating Manual for Prevention and Management of STIs and the National Guidelines for HIV and STI Programming with Key and Vulnerable Populations. He’s been invited to PEPFAR’s COP planning and writing process to develop an anal package of care. Lastly, for World AIDS Day, he published an opinion piece landing in The Nation, The Star and The Standard.
Rhoda Msiska (Zambia) is ensuring a swift introduction of the DPP. She’s earned a leading advocacy role, engaging the Ministry of Health to fast-track and de-medicalize PrEP and ensuring DPP inclusion in the national PrEP implementation plan. She’s working with the MoH to set aside DPP funding through the Global Fund and taking to the radio airwaves to create demand. Importantly, Rhoda has secured a first meeting with ZAMRA, Zambia’s regulatory body, to encourage moving forward with civil society support. And, at R4P Conference in Lima Peru in October, Rhoda spoke on a panel addressing strategies for the delivery of the DPP.
Elina Mwasinga (Malawi) is dually focused on HIV prevention for pregnant women and lactating mothers and HIV cure research. Thus far, she’s secured commitment from the National AIDS Commission to integrate cure into Malawi’s Technical Working Group on Research to coordinate activities and ensure a robust cure research portfolio. Likewise, Elina secured integrated PrEP-family planning services with specified inclusion of pregnant and lactating mothers, as reflected in the MOH’s EMTCT Accountability Roadmap. And, she presented on the use of broadly neutralizing antibodies (bNAbs) to prevent vertical transmission during a WHO consultation and on advocacy as a panelist speaker at the 2024 International AIDS Conference.
Pamela Fuzile (South Africa) is focused on boosting youth engagement in prevention and supporting young champions. She’s establishing a national level youth platform for new technologies in nine provinces where members can influence decisions in the HIV and PrEP technical working groups at SANAC. A key objective is youth advocacy for the inclusion of injectable PrEP and the dapivirine ring in all public health facilities responsive to the needs of young people.
Congrats to all the Fellows on their impressive work thus far. We’ll report on their total accomplishments in late 2025.